Abstract
Background and aims
An important part is played by inflammation in intracranial aneurysm formation. The hypothesis that there is an association of the proinflammatory cytokine interleukin‐6 (IL‐6) genotypes (−572G>C and −174G>C) with intracranial aneurysms was tested.
Methods
IL‐6 genotypes were determined in 91 Caucasian patients with aneurysms and compared with 2720 healthy UK controls.
Results
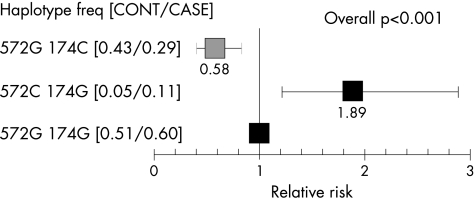
For both polymorphisms, the distribution of the genotypes and estimated allele frequency were different between the control group and the aneurysm group. For −572G>C, a higher frequency of the C allele (p = 0.001) and more people homozygous for the C allele were found among those with aneurysms than among the controls (4.4% v 0.3%, p = 0.001). For −174G>C, more people homozygous for the C allele were found among the controls than among those with aneurysm (18% v 7%, p = 0.007). The 572C/174G haplotype was associated with an increased risk of aneurysms, with the relative risk compared with the common haplotype being 1.89 and that for the −572G/174C haplotype being 0.58 (p<0.0005).
Conclusion
This is the first study to show that IL‐6 promoter polymorphisms are associated with intracranial aneurysmal disease. Whether this association is with the development, progression or rupture of such aneurysms, or represents survivor bias, is unclear.
Although the pathogenesis of cerebral aneurysms is likely to be multifactorial, vessel inflammation is believed to make a substantial contribution.1,2 The pleiotropic cytokine interleukin‐6 (IL‐6) may be essential in this regard, orchestrating the synthesis of the full spectrum of acute‐phase proteins and mediating associated endothelial dysfunction by releasing chemokine and adhesion molecules.
To date, two common functional polymorphisms of the IL‐6 gene promoter have been identified, −572G>C and −174G>C, each consisting of the substitution of a single nucleotide.3,4 Both the −174C and −572C alleles are functional in vitro4,5 and have been associated with greater in vivo IL‐6 synthesis in an inflammatory state.3,5,6 The −174C allele is reported to be associated with higher risk of coronary artery disease in both case–control7 and prospective studies.8 If inflammatory processes indeed play a part in the pathogenesis of intracranial arterial aneurysms, then we can expect the IL‐6 genotype to be similarly associated with aneurysmal disease. Our study examines this hypothesis.
Methods
This study was conducted between February 2002 and October 2003. The study group comprised all consecutive UK Caucasian unrelated patients presenting to the UK National Hospital for Neurology and Neurosurgery with angiographically diagnosed aneurysmal subarachnoid haemorrhage (SAH). The control sample consisted of age‐matched and race‐matched healthy UK people, who were drawn from the second Northwick Park Heart Study9 and previously genotyped for both IL‐6 variants.8 The study was performed with full approval from the institutional ethics committee. Written informed consent was obtained from all participants.
Genetic analysis
The IL‐6 genotype was determined by polymerase chain reaction amplification as described previously.3,4 Positive and negative controls were used to ensure accuracy.
Statistical analysis
Allele frequencies were determined by gene counting, and deviations from the Hardy–Weinberg equilibrium were calculated by using χ2 tests. Differences between the genotype distributions and allele frequencies were calculated by χ2 tests or Fisher's exact tests, as appropriate. Haplotypes were estimated by using a maximum likelihood algorithm10 implemented in the THESIAS program (http://www.genecanvas.ecgene.net/news/php). The haplotype combining the most frequent alleles was used as the reference. A global p value was calculated by using differences in log likelihoods, assuming an additive model of haplotype effects. p Values were adjusted for possible confounders by logistic regression analysis. In addition, differences between people homozygous for the rare alleles and those carrying the common allele were considered. p Values ⩽0.05 were considered to be significant.
Results
Ninety one consecutive Caucasian patients presented during the study period. Table 1 shows the characteristics of the patients and the healthy subjects comparison group. As expected, the patients had a higher prevalence of hypertension but not of diabetes, and a greater proportion were smokers. Although the mean age of the two groups was similar, the range was greater in the patient group.
Table 1 Characteristics of the patient and control populations.
| Patients | Healthy UK subjects | p Value (patients v controls) | |
|---|---|---|---|
| Age | 55 (range 24–80) | 56 (range 49–64) | 0.01 |
| Sex (M:F) | 40:60 | All males | <0.0001 |
| Smoking | 50% | 28.2% | <0.0001 |
| Hypertension* | 51% | 30% | <0.0001 |
| Diabetes | 2% | 2.4% | 0.62 |
| IL‐6 genotype (−174G>C) | |||
| GG/GC/CC, n (%) | 40 (47)/40 (47)/6 (7) | 867 (32)/1358 (50)/495 (18) | 0.003 |
| Frequency of C allele (95% CI) | 0.30 (0.23 to 0.37) | 0.43 (0.42 to 0.44) | 0.001 |
| IL‐6 genotype (−572G>C) | |||
| GG/GC/CC, n (%) | 79 (86.8)/8 (8.8)/4 (4.4) | 2359 (90.4)/244 (9.3)/9 (0.3) | 0.001 |
| Frequency of C allele (95% CI) | 0.09 (0.05 to 0.13) | 0.05 (0.04 to 0.06) | 0.02 |
*Systolic hypertension >160 mm Hg or diastolic hypertension <100 mm Hg.
Genetic analysis
For both variants, the genotype distributions differed significantly between patients and controls (p<0.003; table 1), and this remained significant after adjustment for age, smoking, hypertension and diabetes (p<0.02) and when the analysis was restricted to men (p<0.01).
For −572G>C, the C‐allele frequency was greater among patients than among controls (p = 0.02), with more people homozygous for the C allele among those with aneurysms than among the controls (4.4 v 0.3%, p = 0.001). For −174G>C, genotyping was successful in 86 subjects, whose baseline demographics were in no way different from those in whom genotyping failed (not shown). A greater frequency of the −174C allele was observed in the control group than in the patient group (p = 0.001), with more people homozygous for the C allele among the controls than among those with aneurysms (18% v 7%, p = 0.007).
As reported previously,8 there was strong evidence of negative linkage disequilibrium between the −174G>C and −572G>C polymorphisms, among both the controls (−0.96 δ′, δ −0.19) and the patients. There was significant evidence of a difference in the haplotype distribution between cases and controls (p<0.0005) (fig 1), with the 572C/174G haplotype being associated with an increased risk of aneurysm and the 572G/174C haplotype being protective.
Figure 1 Relative risk of cerebral aneurysms by IL‐6 −572G>C and −174G>C haplotypes (bars are 95% confidence intervals). Haplotype numbers (cases/controls) were −572G/−174G, −572G/−174C and −572C/−174G. The frequency of the −572C/−174C haplotype was extremely low (<0.005) and was not included in the calculation of risk.
Discussion
We report a marked association between both the −174G>C and the −572G>C promoter polymorphisms of the IL‐6 gene and clinical presentation of cerebral aneurysmal disease. The 572C/174G haplotype was found to be associated with an increased risk of aneurysm and the 572G/174C allele with protection from aneurysms. The inflammatory functions of IL‐6 are well known.8 It is now recognised that local IL‐6 synthesis can occur in the brain.11 IL‐6 has been implicated in extracranial aneurysm formation.6,12 Cytokine levels are also predictive of coronary aneurysm formation in Kawasaki disease.13 Similar evidence now exists for intracranial aneurysms. Circulating levels of IL‐6 are raised in patients with ruptured cerebral aneurysms undergoing clipping,14 in both the preoperative and intraoperative situations. Raised levels of IL‐6 were also seen in the internal jugular vein of patients within the first 4 days after SAH and remained raised for up to 14 days.15 The circulating levels appear to be a reflection of local synthesis, and CSF samples have shown increased levels of IL‐6 after aneurysmal SAH.16,17
IL‐6 may also play a part in response to aneurysm rupture. Local IL‐6 is a potent vasoconstrictor of the canine cerebral artery.17 Thus, IL‐6 synthesis in response to SAH may cause the indirect ischaemia associated with vasospasm to become worse. In addition, IL‐6 may cause cerebral injury through more direct means. IL‐6 has powerful cytotoxic effects on cerebral white matter.18 In particular, oligodendrocytes of the periventricular white matter may be particularly vulnerable to the neuro‐cytopathogenic effects of IL‐6 after ischaemia, hypoxic or free radical injury.19 Whether directly or indirectly, IL‐6 probably influences the outcome after SAH. In support of this conjecture, the raised IL‐6 levels associated with SAH correlate strongly with poor prospective neurological outcome.16,17,20
IL‐6 polymorphisms have been studied in the brain in disorders characterised by inflammatory processes. The −174G allele has been associated with Alzheimer's disease.21 Controversy exists about the relationship between the IL‐6 −174 alleles and stroke.22,23 The G allele has also been shown to increase the risk of multi‐infarct dementia.24
Our study has some limitations. Case–control genotype frequency comparisons may be confounded by hidden population stratification. This would be the case in our study if the patient and control groups were composed of different proportions of subjects from different regions of the UK and the frequency of the −572G>C or −174G>C allele was different across the UK. This seems unlikely as there was no marked difference in the frequency of either variant among the nine general practices that made up the Northwick Park Heart Study‐II recruitment sites (not shown), and we have previously reported little, if any, evidence of such population stratification by using a large number of single‐nucleotide polymorphisms throughout the genome.25 Although the Northwick Park Heart Study‐II comprises only men, and the patients include both men and women, a difference in genotype distribution by sex (for an autosomal gene) is unlikely, and the data appear to hold for both single‐nucleotide polymorphisms when confined to male patients.
With any case–control design, however, it is not possible to distinguish between several different mechanisms that could contribute to observed frequency differences. The −572C allele appears to increase the risk of aneurysms, but the lower frequency of the −174C allele may be because it is protective, or because it is associated with other forms of cardiovascular disease. Thus, patients with the −174C allele may be dying from coronary heart disease, as reported previously,7,8 and so the frequency of this genotype will be lower in those who subsequently develop an aneurysm. The frequency of the −174C allele, however, was similar in patients above and below the median age (0.39 v 0.24, p = 0.06), and so this possibility seems less likely. Premorbid mortality for SAH is in the region of 10–15%; hence it must be considered that the cohort of people who were studied in this population are those who have survived the initial insult. Thus, there may be an element of survivor bias.
A final limitation is that we do not have IL‐6 levels for the patient group to explore whether the risk and protective effects are mediated through plasma or tissue levels of this cytokine. Obtaining brain tissue samples is difficult, and it is not clear whether plasma IL‐6 levels either preoperation or postoperation would be directly relevant to this.
In this study, we showed that the IL‐6 −174C and −572C alleles were associated with intracranial aneurysmal disease. Whether this association is with the development, progression or rupture of such aneurysms, or represents survivor bias, is the subject of ongoing research.
Acknowledgements
Mrs Laleh Morgan is funded by the National Hospital Development Foundation, London (Hayward Foundation, Mason Medical Foundation and Peel Medical Research Trust). Dr Hugh Montgomery is funded by the Portex Endowment at the Institute of Child Health, London. This work was supported by a grant to SHE from the British Heart Foundation (PG2000/015) and was carried out in part with support from the Department of Health and the Department of Trade and Industry for the IDEAS Genetics Knowledge Park. We thank Ms Joan Grieve (Consultant Neurosurgeon) and Mr Laurence Watkins (Consultant Neurosurgeon) of the Victor Horsley Department of Neurosurgery, Queen Square, for allowing their patients to be approached for this study, Ms Emma Hawe for help with initial statistical analysis and Prof George Miller for access to the published NPHSII IL‐6 genotype data.
Abbreviations
IL‐6 - interleukin‐6
SAH - subarachnoid haemorrhage
Footnotes
Competing interests: None.
References
- 1.Krex D, Schackert H K, Schackert G. Genesis of cerebral aneurysms—an update. Acta Neurochir (Wien) 2001143429–48 discussion 4489. [DOI] [PubMed] [Google Scholar]
- 2.Sekhar L N, Heros R C. Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery 19818248–260. [DOI] [PubMed] [Google Scholar]
- 3.Brull D J, Montgomery H E, Sanders J.et al Interleukin‐6 gene −174 g>c and −572 g>c promoter polymorphisms are strong predictors of plasma interleukin‐6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol 2001211458–1463. [DOI] [PubMed] [Google Scholar]
- 4.Fishman D, Faulds G, Jeffery R.et al The effect of novel polymorphisms in the interleukin‐6 (IL‐6) gene on IL‐6 transcription and plasma IL‐6 levels, and an association with systemic‐onset juvenile chronic arthritis. J Clin Invest 19981021369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari S L, Ahn‐Luong L, Garnero P.et al Two promoter polymorphisms regulating interleukin‐6 gene expression are associated with circulating levels of C‐reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab 200388255–259. [DOI] [PubMed] [Google Scholar]
- 6.Jones K G, Brull D J, Brown L C.et al Interleukin‐6 (IL‐6) and the prognosis of abdominal aortic aneurysms. Circulation 20011032260–2265. [DOI] [PubMed] [Google Scholar]
- 7.Georges J L, Loukaci V, Poirier O.et al Interleukin‐6 gene polymorphisms and susceptibility to myocardial infarction: the ECTIM study. Etude Cas‐Temoin de l'Infarctus du Myocarde. J Mol Med 200179300–305. [DOI] [PubMed] [Google Scholar]
- 8.Humphries S E, Luong L A, Ogg M S.et al The interleukin‐6 −174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J 2001222243–2252. [DOI] [PubMed] [Google Scholar]
- 9.Miller G J, Bauer K A, Barzegar S.et al Increased activation of the haemostatic system in men at high risk of fatal coronary heart disease. Thromb Haemost 199675767–771. [PubMed] [Google Scholar]
- 10.Tregouet D A, Escolano S, Tiret L.et al A new algorithm for haplotype‐based association analysis: the stochastic‐EM algorithm. Ann Hum Genet 200468(Pt 2)165–177. [DOI] [PubMed] [Google Scholar]
- 11.Ringheim G E, Burgher K L, Heroux J A. Interleukin‐6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin‐6 production in the brain. J Neuroimmunol 199563113–123. [DOI] [PubMed] [Google Scholar]
- 12.Treska V, Topolcan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med 2000381161–1164. [DOI] [PubMed] [Google Scholar]
- 13.Lin C Y, Lin C C, Hwang B.et al Cytokines predict coronary aneurysm formation in Kawasaki disease patients. Eur J Pediatr 1993152309–312. [DOI] [PubMed] [Google Scholar]
- 14.Sablotzki A, Ebel H, Muhling J.et al Dysregulation of immune response following neurosurgical operations. Acta Anaesthesiol Scand 20004482–87. [DOI] [PubMed] [Google Scholar]
- 15.Hirashima Y, Nakamura S, Endo S.et al Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res 1997221249–1255. [DOI] [PubMed] [Google Scholar]
- 16.Gaetani P, Tartara F, Pignatti P.et al Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol Res 199820337–342. [DOI] [PubMed] [Google Scholar]
- 17.Osuka K, Suzuki Y, Tanazawa T.et al Interleukin‐6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir (Wien) 1998140943–951. [DOI] [PubMed] [Google Scholar]
- 18.Yoon B, Kim C, Romero R.et al Amniotic fluid inflammatory cytokines (interleukin‐6, interleukin‐1 beta, and tumour necrosis factor‐a), neonatal brain white matter and cerebral palsy. Am J Obstet Gynecol 199717719–26. [DOI] [PubMed] [Google Scholar]
- 19.Inder T, Volpe J. Mechanisms of perinatal brain injury. Semin Neonatol 200053–16. [DOI] [PubMed] [Google Scholar]
- 20.Ki‐Young K, Byung‐Chan J. Cytokine levels in cerebrospinal fluid and delayed ischemic deficits in patients with aneurysmal subarachnoid hemorrhage. J Korean Med Sci 200116774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pola R, Flex A, Gaetani E.et al The −174 G/C polymorphism of the interleukin‐6 gene promoter is associated with Alzheimer's disease in an Italian population [corrected]. Neuroreport 2002131645–1647. [DOI] [PubMed] [Google Scholar]
- 22.Revilla M, Obach V, Cervera A.et al A −174G/C polymorphism of the interleukin‐6 gene in patients with lacunar infarction. Neurosci Lett 200232429–32. [DOI] [PubMed] [Google Scholar]
- 23.Pola R, Flex A, Gaetani E.et al Synergistic effect of −174 G/C polymorphism of the interleukin‐6 gene promoter and 469 E/K polymorphism of the intercellular adhesion molecule‐1 gene in Italian patients with history of ischemic stroke. Stroke 200334881–885. [DOI] [PubMed] [Google Scholar]
- 24.Pola R, Gaetani E, Flex A.et al −174 G/C interleukin‐6 gene polymorphism and increased risk of multi‐infarct dementia: a case‐control study. Exp Gerontol 200237949–955. [DOI] [PubMed] [Google Scholar]
- 25.Chen X H, Rodriguez S, Hawe E.et al Evidence of admixture from haplotyping in an epidemiological study of UK Caucasian males: implications for association analyses. Hum Hered 200457142–155. [DOI] [PubMed] [Google Scholar]