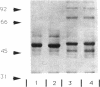
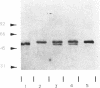
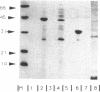
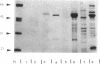
Abstract
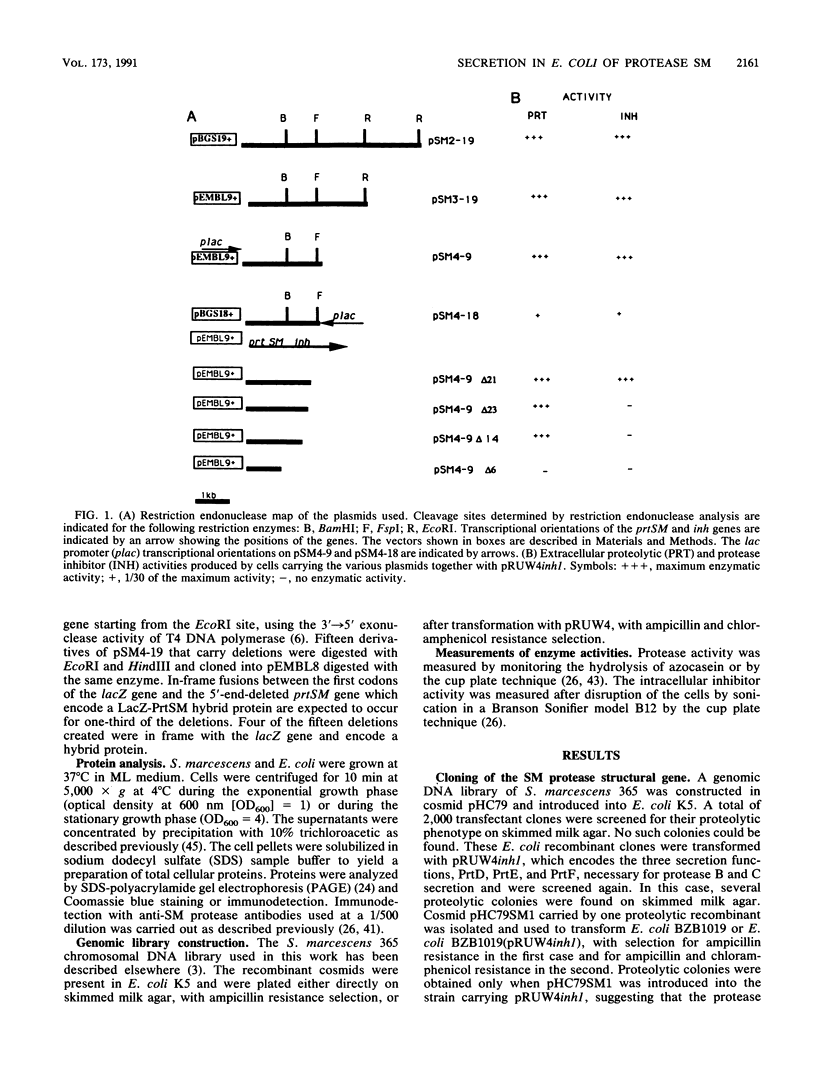
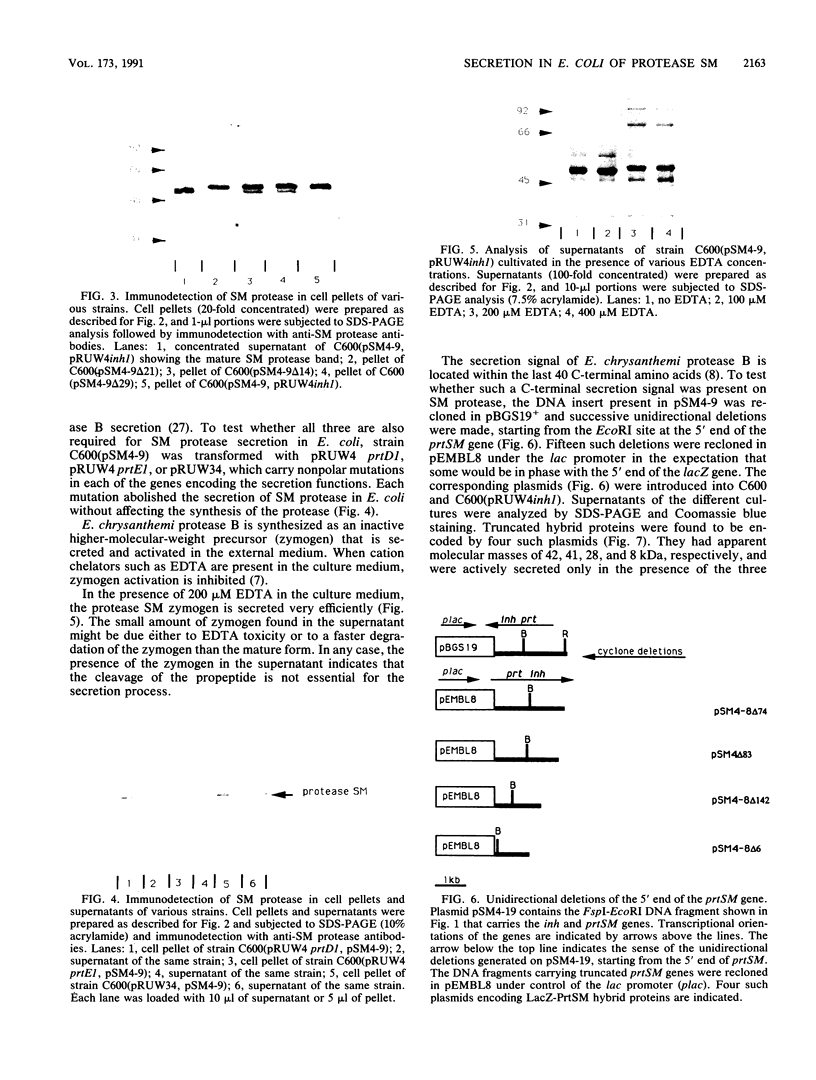
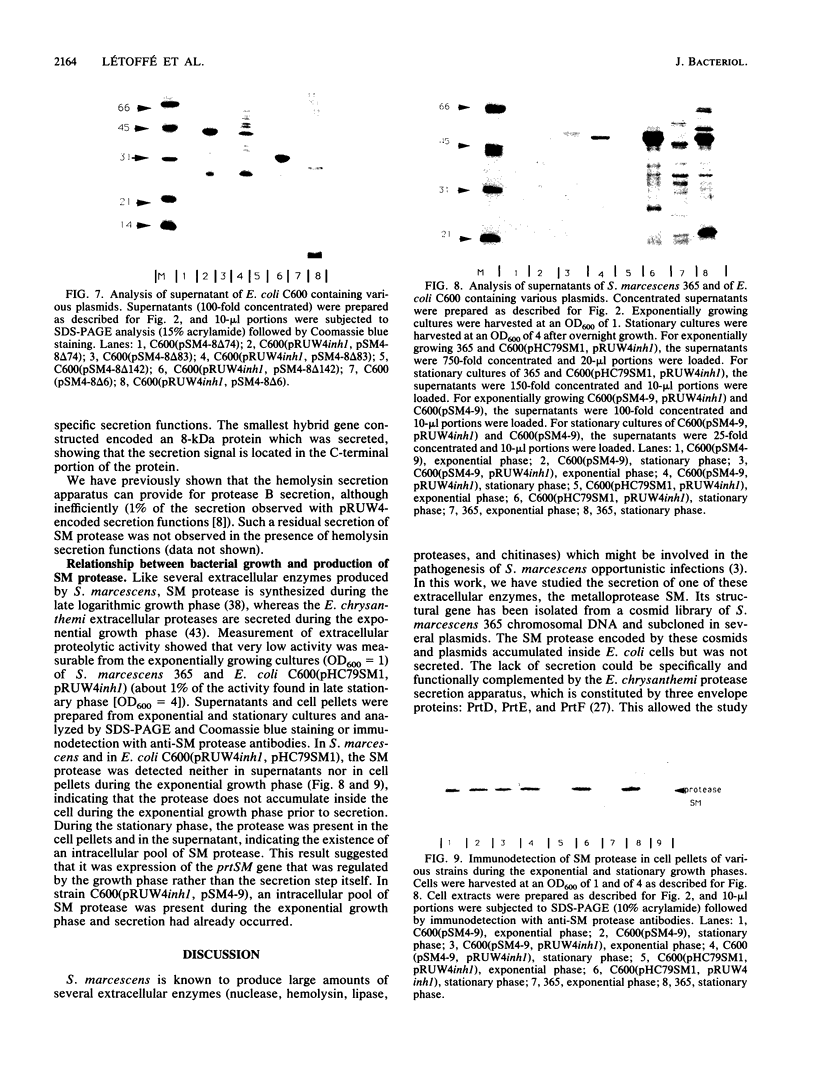
The Serratia marcescens extracellular protease SM is secreted by a signal peptide-independent pathway. When the prtSM gene was cloned and expressed in Escherichia coli, the cells did not secrete protease SM. The lack of secretion could be very efficiently complemented by the Erwinia chrysanthemi protease B secretion apparatus constituted by the PrtD, PrtE, and PrtF proteins. As with protease B and alpha-hemolysin, the secretion signal was located within the last 80 amino acids of the protease. These results indicate that the mechanism of S. marcescens protease SM secretion is analogous to the mechanisms of protease B and hemolysin secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Braun V., Schmitz G. Excretion of a protease by Serratia marcescens. Arch Microbiol. 1980 Jan;124(1):55–61. doi: 10.1007/BF00407028. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1030–1034. doi: 10.1073/pnas.71.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi. Proteases B and C are synthesized and secreted as zymogens without a signal peptide. J Biol Chem. 1989 May 25;264(15):9083–9089. [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protein secretion in gram-negative bacteria. The extracellular metalloprotease B from Erwinia chrysanthemi contains a C-terminal secretion signal analogous to that of Escherichia coli alpha-hemolysin. J Biol Chem. 1990 Oct 5;265(28):17118–17125. [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A., Hamilton W. D., Johnston A. W., Downie J. A. The Rhizobium nodulation gene nodO encodes a Ca2(+)-binding protein that is exported without N-terminal cleavage and is homologous to haemolysin and related proteins. EMBO J. 1990 Feb;9(2):349–354. doi: 10.1002/j.1460-2075.1990.tb08117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A., Bally M., Murgier M., Wretlind B., Lazdunski A. Cloning of xcp genes located at the 55 min region of the chromosome and involved in protein secretion in Pseudomonas aeruginosa. Mol Microbiol. 1989 Feb;3(2):261–265. doi: 10.1111/j.1365-2958.1989.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Garrido M. C., Herrero M., Kolter R., Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988 Jun;7(6):1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson L., Mahanty H. K., Kolter R. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990 Dec;9(12):3875–3884. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L., Baker K., Kenny B., Mackman N., Haigh R., Holland I. B. A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl. 1989;11:45–57. doi: 10.1242/jcs.1989.supplement_11.4. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Chico C., San Millán J. L., Kolter R., Moreno F. Growth phase and ompR regulation of transcription of microcin B17 genes. J Bacteriol. 1986 Sep;167(3):1058–1065. doi: 10.1128/jb.167.3.1058-1065.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. K., Engler M. J., Weinstock G. M. Secretion and expression of the Pasteurella haemolytica Leukotoxin. J Bacteriol. 1990 May;172(5):2343–2350. doi: 10.1128/jb.172.5.2343-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laoide B. M., Ullmann A. Virulence dependent and independent regulation of the Bordetella pertussis cya operon. EMBO J. 1990 Apr;9(4):999–1005. doi: 10.1002/j.1460-2075.1990.tb08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Characterization of a protein inhibitor of extracellular proteases produced by Erwinia chrysanthemi. Mol Microbiol. 1989 Jan;3(1):79–86. doi: 10.1111/j.1365-2958.1989.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J. 1990 May;9(5):1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201(2):282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989 Jul;14(7):268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Neurath H. The versatility of proteolytic enzymes. J Cell Biochem. 1986;32(1):35–49. doi: 10.1002/jcb.240320105. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. The C-terminal, 23 kDa peptide of E. coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) export machinery. FEBS Lett. 1986 Aug 18;204(2):331–335. doi: 10.1016/0014-5793(86)80838-9. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Schmitz G., Braun V. Cell-bound and secreted proteases of Serratia marcescens. J Bacteriol. 1985 Mar;161(3):1002–1009. doi: 10.1128/jb.161.3.1002-1009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P., Letoffe S., Schwartz M. Characterization of Erwinia chrysanthemi extracellular proteases: cloning and expression of the protease genes in Escherichia coli. J Bacteriol. 1987 Nov;169(11):5046–5053. doi: 10.1128/jb.169.11.5046-5053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989 Dec;3(12):1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- d'Enfert C., Ryter A., Pugsley A. P. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 1987 Nov;6(11):3531–3538. doi: 10.1002/j.1460-2075.1987.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]