Abstract
Background
Among elderly people without dementia, the apolipoprotein E ε4 allele (APOE4) has been associated with cognitive deficit, particularly in episodic memory, but few reports are available on whether this association differs by sex.
Methods
In a community‐dwelling Norwegian cohort of 2181 elderly people (55% women), aged 70–74 years, episodic memory was examined in relation to sex and APOE4 zygosity, with the Kendrick Object Learning Test (KOLT).
Results
Possession of at least one APOE4 allele had a modest, detrimental effect on episodic memory in women, whereas in men, heterozygotes were unaffected and homozygotes had markedly lower scores across the distribution of KOLT scores. This sex difference was found consistently in all analyses: on comparing means and medians, examining trends across quintiles, and studying the distribution of scores and the risk of cognitive impairment. Results were broadly similar when adjusted for known determinants of cognition and also when severely impaired participants were excluded. The adjusted odds ratio (OR) of cognitive impairment in women was shown to be 1.8 (95% confidence interval (CI): 1.1 to 2.8) for heterozygotes and 1.1 (0.3 to 3.7) for homozygotes; the adjusted OR in men was observed to be 1.1 (0.6 to 2.1) for heterozygotes and 10.7 (4.7 to 24) for homozygotes.
Conclusions
Although the harmful effect of APOE4 on episodic memory was modest in women, the risk was found to occur in about 30%. APOE4 was observed to have a dramatic effect on episodic memory in men, but only in homozygotes, who comprised about 3% of men: the whole male homozygous group showed a marked shift to lower memory scores.
Age and the apolipoprotein E ε4 allele (APOE4) are the most important known risk factors for sporadic Alzheimer's disease. The disease is thought to have a long presymptomatic phase,1 which suggests that APOE4 starts exerting its detrimental effects in the preclinical phase. Most studies on elderly people without dementia have found that the APOE4 allele is associated with various cognitive deficits,2,3,4,5,6,7,8,9,10,11,12,13,14 particularly in memory.2,3,4,5,6,7 A recent meta‐analysis of more than 20 000 people concluded that this allele was associated with poorer performance on tests of global cognitive functioning, episodic memory and executive functioning.15
The association of APOE4 with Alzheimer's disease varies with sex.16,17,18,19,20 The meta‐analysis by Farrer et al20 found that APOE4 homozygosity affords a high risk of Alzheimer's disease for both men and women, but that a single copy of the allele confers a greater risk on women than on men. A similar sex difference related to APOE4 has been found in the degree of hippocampal atrophy in a cohort with mild cognitive impairment.21 We may therefore expect to find an effect related to sex of the APOE4 allele in cognitive tests in elderly people without dementia. Two studies3,22 that have reported an influence of sex on this relationship found a stronger effect of APOE4 in women.3,22
In this study, we investigated whether sex influences the relationship between APOE alleles and episodic memory in community‐dwelling elderly people. We selected episodic memory because memory deficit is a hallmark of Alzheimer's disease. Tests of episodic memory have been found to be particularly effective in identifying people at risk.23,24 We compared the influence of sex in our cohort with that found on the risk of Alzheimer's disease. We studied a relatively large group of 2181 people from western Norway.
Methods
Study population and data collection
This study was part of the Hordaland Health Study (HUSK), a cross‐sectional population‐based study on chronic diseases in western Norway. The cohort (n = 18 044) was established and first examined in 1992–3. The source population for the cognitive function substudy of HUSK included all people born between 1925 and 1927 and those born between 1950 and 1951, and residing in Bergen and three neighbouring suburban municipalities. The second round of HUSK was conducted from 1997 to 2000; it is described in detail online at http://www.uib.no/isf/husk/Vedlegg_dokumenter/Cognitive_Sub_study.pdf.
At baseline, there were 4110 people born between 1925 and 1927 and living in Bergen, of whom 4093 were genotyped for APOE. Of the 4110 people, 3730 were believed to be still available. They were invited to a second survey conducted from 1997 to 2000 and 2841 took part (follow‐up period 5.2–7.2 years, mean 6.0 years). Of the 77% (2197/2841) who participated in the cognitive function substudy, 2181 had a known APOE genotype. All participants gave their written, informed consent. Table 1 in Nurk et al25 provides detailed characteristics of the study population, (available at http://www3.interscience.wiley.com/cgi‐bin/jissue/112160520).
Table 1 Mean KOLT scores by APOE4 status.
| All | Not severely impaired* | |||
|---|---|---|---|---|
| n | Mean score (SD) | n | Mean score (SD) | |
| Women | ||||
| All | 1197 | 36.7 (8.2) | 1162 | 37.3 (7.4) |
| APOE4 negative | 821 | 37.2 (8.0) | 805 | 37.6 (7.5) |
| APOE4 heterozygotes | 338 | 35.6 (8.4) | 321 | 36.6 (7.2) |
| APOE4 homozygotes | 38 | 34.6 (9.0) | 36 | 35.6 (8.0) |
| p (ANCOVA)† | 0.005‡ | 0.06 | ||
| Men | ||||
| All | 984 | 33.3 (7.9) | 955 | 33.9 (7.3) |
| APOE4 negative | 660 | 33.6 (7.6) | 646 | 34.0 (7.1) |
| APOE4 heterozygotes | 293 | 33.6 (8.0) | 284 | 34.2 (7.4) |
| APOE4 homozygotes | 31 | 24.3 (8.0) | 25 | 26.9 (6.2) |
| p (ANCOVA)† | <0.001§ | <0.001 | ||
ANCOVA, analysis of covariance; APOE4, apolipoprotein E ε4 allele; KOLT, Kendrick Object Learning Test; SD, standard deviation.
*Lowest 3% of KOLT scores by sex (⩽22 for women and ⩽17 for men).
† p Values are after controlling for the covariates listed in the Methods section; mean scores are unadjusted. In analyses with significant results, the following covariates remained in each regression: all women: education, physical activity, HDL (high‐density lipoprotein) cholesterol, triglycerides and homocysteine; all men: education, physical activity, diastolic blood pressure, HDL cholesterol, non‐HDL cholesterol, homocysteine and history of cardiovascular disease or hypertension; men not severely impaired: education, physical activity, diastolic blood pressure and homocysteine.
‡Groupwise heterozygotes versus negatives, p = 0.003; homozygotes versus negatives, p = 0.08; homozygotes versus heterozygotes, p = 0.65.
§Groupwise heterozygotes versus negatives, p = 0.8; homozygotes versus negatives, p<0.001; homozygotes versus heterozygotes, p<0.001.
The baseline and follow‐up examinations included data on lifestyle, medical history and cardiovascular risks. Blood samples were collected for assessment of cardiovascular risk factors and homocysteine‐related variables on both occasions. The details of data collection were described previously.25,26 Genotyping for APOE was carried out by a standard polymerase chain reaction method on the blood samples that were collected at baseline and stored frozen.27
Cognitive testing
The follow‐up examination included the Kendrick Object Learning Test (KOLT). The KOLT was designed to assess dementia status and memory performance among elderly people who were not institutionalised.28 Four cards with 10, 15, 20 and 25 pictures were shown individually for 30, 45, 60 and 75 s. Then the participants were asked to name as many pictures on the card as they could remember. The maximum score was 70. A score of ⩽20 indicated severe cognitive impairment or dementia, whereas a score of 21–25 indicated moderate impairment. The KOLT has been validated for the detection of both mental impairment in old age29 and mild Alzheimer's disease.30 It has been widely used in Norway for 20 years.
Statistical analyses
We examined the interaction between sex and APOE4 status in the prediction of the KOLT score by using linear regression analysis. Then, for each sex, we compared KOLT scores by APOE4 status with analysis of covariance, followed by groupwise comparisons, if significant. Linear regression was used to assess the dose relationship between APOE4 zygosity and KOLT score. We compared proportions of APOE4 heterozygotes and homozygotes by quintile of KOLT score with the linear test for trend. We defined cognitive impairment as the lowest decile of the KOLT score in each sex and severe cognitive impairment as the lowest 3% of the KOLT score by sex. We then repeated the above analyses, omitting those with severe impairment (lowest 3%). We also estimated the odds ratios (ORs) and 95% confidence intervals (CIs) of cognitive impairment (lowest decile) for heterozygotes and homozygotes, taking participants without APOE4 as reference, by Fisher's exact test and logistic regression. Potential sex differences in KOLT scores by APOE2 status were also studied in analyses by means, by quintiles and by risk of cognitive impairment.
In analysis of covariance and linear and logistic regression analyses, we controlled for baseline variables where available; otherwise, follow‐up data were used—for example, for KOLT scores and serum creatinine. In the initial models, we controlled for age, education (five categories: “primary school” to “university”), physical activity (four categories: “sedentary” to “competitive sports several times a week”), systolic and diastolic blood pressure, smoking (number of cigarettes a day), history of diabetes, history of cardiovascular disease or hypertension, and plasma or serum concentrations of homocysteine, high‐density lipoprotein (HDL) cholesterol, non‐HDL cholesterol, triglycerides and creatinine. We then progressively removed covariables with higher p values until all remaining values were p<0.2. Results of those final models are quoted later in the article. Normal distribution of the KOLT score was assessed using the Kolmogorov–Smirnov D Goodness‐of‐Fit Test, whereas the location of the distribution was determined by comparing medians (Mann–Whitney U test) and logistic regression analyses, estimating the OR for a low or high KOLT score by using a series of cut‐off points (on the 10, 20, 40, 60, 80 and 90th centile), as described previously.31,32 Values of p<0.05 were considered to be significant.
Results
In both men and women, APOE genotypes were in Hardy–Weinberg equilibrium. The APOE4 allelic frequency was 17.3% in the 1197 women (mean age 72.5 years) and 18.0% in the 984 men (mean age 72.5 years). To investigate possible selection bias, we compared the 4093 participants from the baseline cohort in 1992–3, with those who were invited again during 1997–9, those who attended follow‐up and those who underwent cognitive testing. We also examined those who dropped out at any stage. APOE4‐positive women attending follow‐up were fewer than those not attending (p<0.001). Among those attending follow‐up, there was a tendency for more APOE4‐positive women in those who participated in the cognitive function substudy (p = 0.08). No significant difference by sex was observed in APOE4 status between those cognitively tested and those from the original Bergen cohort not tested.
KOLT
In all, 236 of 2181 (10.8%) participants were cognitively impaired, as defined by a cut‐off of ⩽25 on the KOLT, of whom 74 (3.4%) fulfilled the criteria for dementia by this test (KOLT ⩽20). As previously reported from this cohort,25 women outperformed men on the KOLT (mean score 36.7 v 33.3; p<0.001; t test). Because of this difference, we defined sex‐specific cut‐offs for cognitive impairment and for severe impairment as the lowest decile and the lowest 3%, respectively, of the KOLT score for each sex.
Linear regression analysis indicated significant interactions between sex and APOE4 status in the prediction of KOLT score, whether comparing APOE4 heterozygotes with APOE4 negatives (p = 0.03 for the interaction with sex) or homozygotes with negatives (p<0.001 for the interaction with sex). We controlled for the covariates listed in the Methods section, of which education, physical activity, homocysteine, HDL cholesterol and history of cardiovascular disease or hypertension remained in the regression in the comparison of heterozygotes with negatives, and age, education, physical activity, homocysteine, HDL cholesterol, triglycerides and diabetes in the comparison of homozygotes with negatives. All further analysis was conducted separately by sex.
In both sexes, significant differences were observed in the KOLT score among the three groups according to APOE4 zygosity (table 1). Among women, both APOE4 heterozygotes and homozygotes had lower KOLT scores than those without the allele, but only the heterozygotes were significantly different. For the heterozygotes, the difference in mean KOLT score remained significant after controlling for the covariates listed in the methods section (p = 0.003). A significant linear trend was observed in KOLT scores by number of APOE4 alleles (p = 0.002) after adjustment for the covariates. Among men, APOE4 heterozygotes had KOLT scores almost identical to those without the allele. APOE4 homozygotes, however, had a much lower mean score than each of the other groups (p<0.001) after controlling for the listed covariates.
Quintiles of KOLT scores
Among women, the proportion of APOE4 alleles increased with decreasing quintiles of KOLT score (p = 0.009, linear test for trend, after controlling for the listed covariates), as did that of APOE4 heterozygotes (table 2). The proportion of APOE4 homozygotes showed a modest, non‐significant increase. Among men, the proportion of APOE4 alleles increased with decreasing quintiles of KOLT score (p = 0.007), which was entirely due to a striking increase in the proportion of homozygotes in the lower quintiles (table 2). By contrast, there was no consistent change in the proportion of male heterozygotes.
Table 2 APOE4 status by quintiles of KOLT scores.
| p (trend)* | ||||||
|---|---|---|---|---|---|---|
| Women | ||||||
| Quintile (score) | 1 (0–29) | 2 (30–34) | 3 (35–38) | 4 (39–43) | 5 (44–65) | |
| n | 218 | 262 | 214 | 262 | 241 | |
| Proportions (%) | ||||||
| APOE4 negatives | 63.3 | 65.3 | 68.7 | 71.8 | 73.4 | 0.009 |
| Heterozygotes | 31.7 | 31.3 | 28.0 | 26.3 | 24.1 | 0.04 |
| Homozygotes | 5.0 | 3.4 | 3.3 | 1.9 | 2.5 | 0.2 |
| Men | ||||||
| Quintile (score) | 1 (0–26) | 2 (27–31) | 3 (32–35) | 4 (36–40) | 5 (41–61) | |
| n | 192 | 211 | 197 | 201 | 183 | |
| Proportions (%) | ||||||
| APOE4 negatives | 61.5 | 69.7 | 71.1 | 63.2 | 69.9 | 0.3 |
| Heterozygotes | 29.2 | 27.0 | 26.9 | 36.3 | 29.5 | 0.4 |
| Homozygotes | 9.4 | 3.3 | 2.0 | 0.5 | 0.5 | <0.001 |
APOE4, apolipoprotein E ε4 allele; KOLT, Kendrick Object Learning Test.
*Controlling for the covariates listed in the Methods section, of which the following remained in each regression which had a significant result: women, APOE4 negative: age, education, physical activity, high‐density lipoprotein (HDL) cholesterol and triglycerides; women, APOE4 heterozygotes: education, physical activity, HDL cholesterol, triglycerides and history of cardiovascular disease or hypertension; men, APOE4 homozygotes: education, physical activity, diastolic blood pressure, HDL cholesterol, non‐HDL cholesterol and homocysteine.
Excluding those with severe cognitive impairment
We repeated the above analyses, omitting participants with severe cognitive impairment (lowest 3% of KOLT score—that is, ⩽22 for women, ⩽17 for men). This only weakened the results slightly (table 1). Among women, the difference in mean KOLT score between APOE4 heterozygotes and those without APOE4 became less, but was still significant, in both unadjusted (p = 0.025) and adjusted analyses (p = 0.035). All significant trends by quintiles of score (table 2) remained significant. Among men, all significant results remained significant—for example, the lower score in APOE4 homozygotes (p<0.001), in both unadjusted and adjusted analyses (table 1) and the increasing proportion of homozygotes with lower quintile of score (p<0.001; table 2).
Risk of cognitive impairment
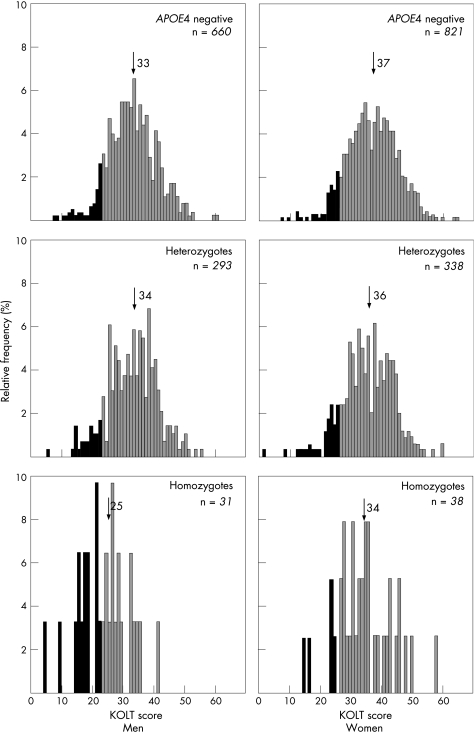
About 13% of women among both APOE4 heterozygotes and homozygotes were cognitively impaired (cut‐off at the lowest decile of KOLT scores for women was ⩽26; fig 1). Close to 10% of APOE4 heterozygous men were cognitively impaired (cut‐off at the lowest decile of KOLT score was ⩽23), whereas the proportion of cognitively impaired men was more than 45% among homozygotes (nearly five times higher; fig 1).
Figure 1 Frequency distribution of the Kendrick Objective Learning Test (KOLT) scores in men and women in relation to APOE4 status. Filled columns indicate those who were cognitively impaired, using the sex‐specific cut‐off values of ⩽26 for women and ⩽23 for men. Median values for each distribution are indicated by an arrow.
In women, taking those without APOE4 as reference, heterozygotes were at increased risk of cognitive impairment, whereas this association was not significant in homozygotes (table 3). By contrast, among men, heterozygotes were not at increased risk of cognitive impairment compared with men without APOE4, whereas homozygotes were at greatly increased risk (table 3).
Table 3 Risk of cognitive impairment according to KOLT by APOE4 status.
| OR* of cognitive impairment† (95% CI) | ||||
|---|---|---|---|---|
| Impaired (n) | Unimpaired (n) | |||
| Unadjusted | Adjusted‡ | |||
| Women | ||||
| APOE4 negatives | 72 | 749 | Reference | Reference |
| APOE4 heterozygotes | 44 | 294 | 1.6 (1.05 to 2.3) | 1.8 (1.1 to 2.8) |
| APOE4 homozygotes | 5 | 33 | 1.6 (0.6 to 4.2) | 1.1 (0.3 to 3.7) |
| Men | ||||
| APOE4 negatives | 51 | 609 | Reference | Reference |
| APOE4 heterozygotes | 28 | 265 | 1.3 (0.8 to 2.0) | 1.1 (0.6 to 2.1) |
| APOE4 homozygotes | 14 | 17 | 9.8 (4.6 to 21) | 10.7 (4.7 to 24) |
APOE4, apolipoprotein E ε4 allele; CI, confidence interval; KOLT, Kendrick Objective Learning Test; OR, odds ratio.
*Compared with participants without APOE4.
†Lowest decile by sex.
‡For the covariates listed in the Methods section, of which the following remained in each regression: women, APOE4 heterozygotes: education, high‐density lipoprotein cholesterol, triglycerides, history of cardiovascular disease or hypertension; women, APOE4 homozygotes: history of cardiovascular disease or hypertension; men, APOE4 heterozygotes: education, systolic blood pressure, creatinine, history of cardiovascular disease or hypertension; men, APOE4 homozygotes: education.
Distribution of KOLT scores in relation to APOE4 zygosity
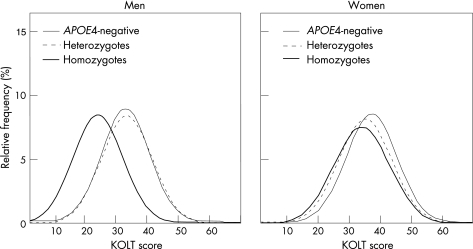
Figure 1 shows the distributions of KOLT scores according to APOE4 zygosity and sex. The scores were consistent with normal distribution in all groups in both sexes (p>0.05, Kolmogorov–Smirnov test). Figure 2 shows that the distribution of KOLT scores in male APOE4 homozygotes was uniformly shifted to lower values, suggesting that their low mean score (table 1) was not explained by a subgroup with particularly low scores. In men, the median score of APOE4 homozygotes (fig 1) was lower than those of APOE4‐negative participants (p<0.001) and heterozygotes (p<0.001). Examining a range of cut‐offs (see Methods section), the ORs for having low KOLT scores were 6–10 times higher, whereas the ORs for having high KOLT scores were at least eight times lower in homozygous men than in the APOE4‐negative group. Thus in men, APOE4 homozygosity influenced high KOLT scores just as much as, or more than, low KOLT scores. For heterozygous men, there was no significant change in the location of the distribution compared with the APOE4‐negative group (fig 2). In women, significant differences were found between the median KOLT scores of APOE4 negatives and heterozygotes (p = 0.008) or homozygotes (p = 0.044). In female heterozygotes and homozygotes, the effect on either tail of the distribution was small and similar in both groups and was only borderline significant, but here again, the analyses showed that APOE4 induced a shift in distribution from higher to lower values. The ORs for having low KOLT scores were 1.3–2 times higher, whereas the ORs for having high KOLT scores were 1.3–2 times lower, in homozygous and heterozygous women, than in APOE4‐negative women (fig 2).
Figure 2 Normal frequency distributions of the Kendrick Objective Learning Test (KOLT) scores in men and women in relation to APOE4 status. The normal distributions were generated from the observed frequency distributions shown in fig 1.
Effect of APOE2
Analysis by means, or by quintiles, or by risk of cognitive impairment did not show any significant effect of APOE2, by either genotype or allele, on KOLT scores in women (data not shown). But there was a tendency for women who were heterozygous for APOE2 to be at lower risk of severe impairment: OR 0.2 (95% CI 0.03 to 1.4; p = 0.075). No significant influence of APOE2 on KOLT scores was detected in men (data not shown).
Discussion
In this study of a relatively large cohort of community‐based elderly participants, APOE4 was associated with poorer performance on a test of episodic memory in both sexes, but we found marked sex differences. In women, the effect was relatively modest, with an increase of 80% in the risk for cognitive impairment among APOE4 heterozygotes, who comprised almost 30% of the female population tested. By contrast, in men the increased risk was much greater at 10‐fold, but this applied to only 3% of the male population—that is, the APOE4 homozygotes. Nearly half the male homozygotes were cognitively impaired and the entire distribution of scores was shifted to lower values. These differences by sex were consistently seen in all analyses. The analyses by quintiles (table 2) and the location of distribution (fig 2) showed that these effects in both sexes were present at all levels of the KOLT score, not just in the cognitively impaired.
Comparisons with the risk for Alzheimer's disease
Impairment in episodic memory is a cardinal feature of Alzheimer's disease and is detectable well before the onset of clinically diagnosed dementia.33,34 Our results with the KOLT, a test of episodic memory, are consistent with the sex‐specific associations of APOE4 with Alzheimer's disease, in which a greater risk among heterozygotes has been found in women than in men.16,17,18,19,20 In particular, the fact that we could not detect any difference in KOLT scores between male APOE4 heterozygotes and men without the APOE4 allele is consistent with findings that male APOE4 heterozygotes, compared with men with the ε3/ε3 genotype, show little or no increased risk for Alzheimer's disease.16,18,19,20 Likewise, our finding of a strong effect of homozygosity in men is very similar to the strong influence of homozygosity on the risk for Alzheimer's disease in men.20 Our results also reflect those seen in the influence of sex on the associations of APOE4 with hippocampal atrophy in participants with mild cognitive impairment.21
Comparisons with other studies in elderly people without dementia
More than 100 studies have examined the association of APOE4 with cognitive or functional impairment in elderly people without dementia. Most have found a detrimental effect of APOE42,3,4,5,6,7,8,9,10,11,12,13,14,15 on cognition and many included a test of episodic memory.2,3,4,5,6,7,15 Three studies35,36,37 reported no effect of APOE4 on episodic memory, although only one of these37 specifically looked for an interaction between APOE4 and sex but was too underpowered to detect such an effect. One report,3 however, suggested that the effect of APOE4 on episodic memory may be stronger in women. In the meta‐analysis by Small et al,15 the effect of heterozygosity was <0.1 SD unit in each cognitive domain, including episodic memory. In female heterozygotes, we found a difference of 0.2 SD in the KOLT score, whereas in male heterozygotes there was no difference. Therefore, in most studies, including Small et al's meta‐analysis, where sex was not taken into account, the small but significant effect of APOE4 in female heterozygotes would have been diluted by the negligible effect in men.
A second finding in our cohort was the effect of zygosity. The influence of APOE4 zygosity on cognition has been rather rarely studied,7,8,10,14,15 and the sex contrast in such an effect has not been reported. We were in a position to study this effect, partly because of our relatively large number of APOE4 homozygotes (69/2181 = 3.2%), due to the high allelic frequency in Norwegians.38 Among other large studies, we are aware of only one39 that had more homozygotes than our cohort. After controlling for covariates, we found an allelic dose effect in the association of APOE4 with KOLT scores in women (p = 0.002), consistent with the results of two large studies7,10 that were confined to women. Our most striking finding in relation to zygosity was in men, where heterozygotes performed as well as APOE4‐negative participants, whereas homozygotes had markedly lower scores. The effect size for cognitive impairment in male homozygotes was 1.2 SD units, more than 20 times that found for episodic memory in the meta‐analysis by Small et al,15 where the two sexes were combined. Almost half the male homozygotes (14/31) were cognitively impaired. Notably, as is apparent from fig 2, the effect on the KOLT score in homozygous men is explained by a complete shift of the distribution towards lower KOLT scores, not by a few severely affected people. Thus it appears that APOE4 homozygosity harms episodic memory in all men, leading to a KOLT score of about 8–9 points below that for other men. A previous study8 that was confined to men also reported a strong effect of APOE4 homozygosity, but relatively little effect of heterozygosity, on the decline in Mini‐Mental State Examination score over time. In men who are homozygous for APOE4, the effect on KOLT scores is dramatic, but it seems to be an expression of their normal phenotype. A critical question is how early the effect of APOE4 homozygosity in men is expressed or indeed whether it represents an inborn characteristic. In support of its being an inborn characteristic, an influence of APOE4 on memory has been reported in a much younger cohort, with a mean age of 46 (range 24–60) years.40 A unique cohort study41 showed that APOE4 was associated with poor performance on an intelligence test at age 80, whereas it was not so associated in the same participants tested when they were 11 years old. The study, however, did not have the power to detect an effect limited to male homozygotes.
Strengths and limitations
One strength of our study is the size of the cohort, which gave us a relatively large number of homozygotes, and the narrow age range, which overcame possible effects of age on the relationship between APOE4 and memory.15 Furthermore, we had data on many candidate risk factors for cognitive decline and were able to control for these. Another strength is that we used the KOLT. This test was chosen because APOE4 has been particularly associated with loss of episodic memory and delayed recall,2,3,4,5,6,7,15 and such a loss is one of the earliest clinical signs of impending Alzheimer's disease.33,34 It is possible that our results may be specific to episodic memory, which may be why they reflect the sex‐related and zygosity‐related associations found for APOE4 with the risk for Alzheimer's disease.16,17,18,19,20 Although there are numerous tests for episodic memory, the KOLT is one that displays a normal distribution, shows no ceiling effect and has high sensitivity and specificity in the detection of mild Alzheimer's disease.30
A limitation of this study is the lack of longitudinal data on cognition. Studies on APOE4 in elderly people may be affected by selective mortality, but the allelic frequency in our study was still around 18%, compared with nearly 20% in a Norwegian population study.38 Therefore, selective mortality probably did not influence our results much, if at all. We have previously reported that there were differences in a variety of parameters between attenders and non‐attenders at follow‐up, and between those who did or did not undergo cognitive testing;25 the differences in relation to APOE4 status have been described in the Results section. We have adjusted for these factors in our analyses and, overall, we consider it unlikely that these biases could explain the different patterns obtained in men and in women, nor the dramatic effect on episodic memory in male APOE4 homozygtes. No significant difference by sex was seen in APOE4 status between those taking part in this study and those from the original Bergen cohort not taking part.
Conclusions
The striking sex contrast in the association of APOE4 with impairment of episodic memory in a large community cohort is consistent with the sex differences found in relation to the risk for Alzheimer's disease.16,17,18,19,20 In women, we find that the effect of APOE4 is modest and that heterozygotes—that is, about 30% of women—are at risk, whereas in men those (about 3%) who are homozygous for APOE4 are affected, with a marked shift to lower cognitive test scores across the whole group. These observations suggest that APOE4 may exhibit a different pathology in men and in women. Our results have generated a hypothesis that needs confirmation. One way forward is a new meta‐analysis in which the results of primary data, particularly on episodic memory, are analysed separately for the two sexes. If future work confirms our findings, it should stimulate the search for sex‐related factors that could modify the effect of APOE4, and perhaps provide a strategy for improving memory in general and for prevention or slowing of cognitive decline induced by APOE4. Finally, our results emphasise the need for sex‐specific analysis of complex traits.
Acknowlegements
We thank the Norman Collisson Foundation and the Advanced Research Programme of Norway for financial support.
Abbreviations
APOE4 - apolipoprotein E ε4 allele
CI - confidence interval
HDL - high‐density lipoprotein
HUSK - Hordaland Health Study
KOLT - Kendrick Object Learning Test
OR - odds ratio
Footnotes
Competing interests: None.
Ethical approval: The Regional Committee for Medical Research Ethics of western Norway approved the study protocol.
Data collection was conducted as part of the Hordaland Health Study (HUSK) 1997–9, as a collaboration between the University of Bergen, the Norwegian National Health Screening Service (now the Norwegian Institute of Public Health) and local health services. The project was partially financed with support from the Research Council of Norway. Dr EN held a Blaschko Visiting Research Scholarship at the University of Oxford.
References
- 1.Ohm T G, Müller H, Braak H.et al Close‐meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer's disease‐related neurofibrillary changes. Neuroscience 199564209–217. [DOI] [PubMed] [Google Scholar]
- 2.Helkala E L, Koivisto K, Hanninen T.et al Memory functions in human subjects with different apolipoprotein E phenotypes during a 3‐year population‐based follow‐up study. Neurosci Lett 1996204177–180. [DOI] [PubMed] [Google Scholar]
- 3.Hyman B T, Gomez‐Isla T, Briggs M.et al Apolipoprotein E and cognitive change in an elderly population. Ann Neurol 19964055–66. [DOI] [PubMed] [Google Scholar]
- 4.Mayeux R, Small S A, Tang M.et al Memory performance in healthy elderly without Alzheimer's disease: effects of time and apolipoprotein‐E. Neurobiol Aging 200122683–689. [DOI] [PubMed] [Google Scholar]
- 5.Wilson R S, Schneider J A, Barnes L L.et al The apolipoprotein E ε4 allele and decline in different cognitive systems during a 6‐year period. Arch Neurol 2002591154–1160. [DOI] [PubMed] [Google Scholar]
- 6.Deary I J, Whiteman M C, Pattie A.et al Apolipoprotein E gene variability and cognitive functions at age 79: a follow‐up of the Scottish mental survey of 1932. Psychol Aging 200419367–371. [DOI] [PubMed] [Google Scholar]
- 7.Kang J H, Logroscino G, De Vivo I.et al Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging 200526475–484. [DOI] [PubMed] [Google Scholar]
- 8.Feskens E J M, Havekes L M, Kalmijn S.et al Apolipoprotein e4 allele and cognitive decline in elderly men. BMJ 19943091202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berr C, Dufouil C, Brousseau T.et al Early effect of ApoE‐ε4 allele on cognitive results in a group of highly performing subjects: the EVA study. Neurosci Lett 19962189–12. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Cauley J, Sands L.et al Apolipoprotein E phenotype and cognitive decline in a prospective study of elderly community women. Arch Neurol 1997541110–1114. [DOI] [PubMed] [Google Scholar]
- 11.Kuller L H, Shemanski L, Manolio T.et al Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 199829388–398. [DOI] [PubMed] [Google Scholar]
- 12.Fillenbaum G G, Landerman L R, Blazer D G.et al The relationship of APOE genotype to cognitive functioning in older African‐American and Caucasian community residents. J Am Geriatr Soc 2001491148–1155. [DOI] [PubMed] [Google Scholar]
- 13.Tilvis R S, Kahonen‐Vare M H, Jolkkonen J.et al Predictors of cognitive decline and mortality of aged people over a 10 year period. J Gerontol A Biol Sci Med Sci 200459268–274. [DOI] [PubMed] [Google Scholar]
- 14.Shadlen M ‐ F, Larson E B, Wang L.et al Education modifies the effect of apolipoprotein epsilon 4 on cognitive decline. Neurobiol Aging 20052617–24. [DOI] [PubMed] [Google Scholar]
- 15.Small B J, Rosnick C B, Fratiglione L.et al Apolipoprotein E and cognitive performance: a meta‐analysis. Psychol Aging 200419592–600. [DOI] [PubMed] [Google Scholar]
- 16.Poirier J, Davignon J, Bouthillier D.et al Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993342697–699. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda M, Maeda K, Shimada K.et al Apolipoprotein E ε4 allele and gender difference in risk of Alzheimer's disease. Alzheimer's Res 1995177–81. [Google Scholar]
- 18.Payami H, Zareparsi S, Montee K R.et al Gender difference in apolipoprotein E‐associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 199658803–811. [PMC free article] [PubMed] [Google Scholar]
- 19.Farrer L A, Cupples L A, van Duijn C M.et al Apolipoprotein E genotype in patients with Alzheimer's disease: implications for the risk of dementia among relatives. Ann Neurol 199538797–808. [DOI] [PubMed] [Google Scholar]
- 20.Farrer L A, Cupples L A, Haines J L.et al Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta‐analysis. JAMA 19972781349–1356. [PubMed] [Google Scholar]
- 21.Fleisher A, Grundman M, Jack C R J.et al Sex, apolipoprotein E ε4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 200562953–957. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen E L, Høgh P. A gender difference in the association between APOE genotype and age‐related cognitive decline. Neurology 20015789–95. [DOI] [PubMed] [Google Scholar]
- 23.Bäckman L, Jones S, Berger A K.et al Cognitive impairment in preclinical Alzheimer's disease: a meta‐analysis. Neuropsychology 200519520–531. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal N T, Wilson R S, Beck T L.et al Mild cognitive impairment in different functional domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005761479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurk E, Refsum H, Tell G S.et al Plasma total homocysteine and memory in the elderly. The Hordaland Homocysteine Study. Ann Neurol 200558847–857. [DOI] [PubMed] [Google Scholar]
- 26.Nygård O, Vollset S E, Refsum H.et al Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA 19952741526–1533. [DOI] [PubMed] [Google Scholar]
- 27.Wenham P R, Price W H, Blundell G. Apolipoprotein E genotyping by one‐stage PCR. Lancet 19913371158–1159. [DOI] [PubMed] [Google Scholar]
- 28.Kendrick D C, Gibson A J, Moyes I C A. The revised Kendrick battery: clinical studies. Br J Soc Clin Psychol 197918329–340. [DOI] [PubMed] [Google Scholar]
- 29.Engedal K, Haugen P K, Gilje K.et al Efficacy of short mental tests in the detection of mental impairment in old age. Compr Gerontol 1988287–93. [PubMed] [Google Scholar]
- 30.des Rosiers G, Hodges J R, Berrios G. The neuropsychological differentiation of patients with very mild Alzheimer's disease and/or major depression. J Am Geriatr Soc 1995431256–1263. [DOI] [PubMed] [Google Scholar]
- 31.Refsum H, Nygård O, Kvåle G.et al The Hordaland Homocysteine Study: the opposite tails odds ratios reveal differential effects of gender and intake of vitamin supplements at high and low plasma total homocysteine concentrations. J Nutr 19961261244S–1248S. [DOI] [PubMed] [Google Scholar]
- 32.Nygård O, Refsum H, Ueland P M.et al Coffee consumption and plasma total homocysteine: the Hordaland Homocysteine Study. Am J Clin Nutr 199765136–143. [DOI] [PubMed] [Google Scholar]
- 33.Bäckman L, Small B J, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain 200112496–102. [DOI] [PubMed] [Google Scholar]
- 34.Elias M F, Beiser A, Wolf P A.et al The preclinical phase of Alzheimer disease: a 22‐year prospective study of the Framingham cohort. Arch Neurol 200057808–813. [DOI] [PubMed] [Google Scholar]
- 35.Smith G E, Bohac D L, Waring S C.et al Apolipoprotein E genotype influences cognitive ‘phenotype' in patients with Alzheimer's disease but not in healthy control subjects. Neurology 199850355–362. [DOI] [PubMed] [Google Scholar]
- 36.Small B J, Graves A B, McEvoy C L.et al Is APOE‐ε4 a risk factor for cognitive impairment in normal aging? Neurology 2000542082–2088. [DOI] [PubMed] [Google Scholar]
- 37.Kim K W, Youn J C, Jhoo J H.et al Apolipoprotein E ε4 allele is not associated with the cognitive impairment in community‐dwelling normal elderly individuals. Int J Geriatr Psychiatry 200217635–640. [DOI] [PubMed] [Google Scholar]
- 38.Kumar T, Liestol K, Maehlen J.et al Allele frequencies of apolipoprotein E gene polymorphisms in the protein coding region and promoter region (‐491A/T) in a healthy Norwegian population. Hum Biol 200274137–142. [DOI] [PubMed] [Google Scholar]
- 39.Haan M N, Shemanski L, Jagust W J.et al The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 199928240–46. [DOI] [PubMed] [Google Scholar]
- 40.Flory J D, Manuck S B, Ferrell R E.et al Memory performance and the apolipoprotein E polymorphism in a community sample of middle‐aged adults. Am J Med Genet 200096707–711. [DOI] [PubMed] [Google Scholar]
- 41.Deary I J, Whiteman M C, Pattie A.et al Cognitive change and the APOE ε4 allele. Nature 2002418932. [DOI] [PubMed] [Google Scholar]