Niemann–Pick disease type C (NPC) is an autosomal recessive neurovisceral lipid storage disease resulting from mutations of either the NPC1 (95% of families) or the NPC2 gene, showing a wide spectrum of clinical phenotypes and a highly variable age at diagnosis.1,2
We report NPC1 in the oldest patient affected with the disease so far. This 68‐year‐old woman presented with a 15‐year history of depression and fluctuating mood, and was treated several times in psychiatric departments during the previous years. At the age of 54 she was unable to work further. In the past 4 years she had developed a fluctuating, progressive dementia with reduced impulse, affective instability, dysphagia, cramped hands and dyskinesia. A percutaneous endoscopic gastrostomy was initiated owing to the loss of considerable body weight in the previous months. Initial examination, 4 years ago, showed blepharospasm, a vertical gaze palsy and choreiform oral‐buccal movements in the patient. She was bedridden and was not able to communicate, was only intermittently groaning and followed simple requests inconstantly. Her hands were held in a dystonic, flectional position and her upper extremities were moved stereotypically. A positive bilateral Babinski sign was found. Cerebral MRI showed a mild cerebellar atrophy and some isolated periventricular signal intensities in the T2/TIRM. Protein was slightly enhanced (73.7 mg/dl) in CSF, without pleocytosis. Initial electroencephalography showed only a mild slowing. CT of the abdomen showed a mild splenomegaly (14 cm), which was unknown before, and a hepatic steatosis.
Owing to the combination of a slowly progredient neurodegenerative disorder with splenomegaly and vertical gaze palsy, NPC was suspected and confirmed in skin fibroblasts, where filipin staining showed a clearcut pattern of the variant biochemical subtype.1
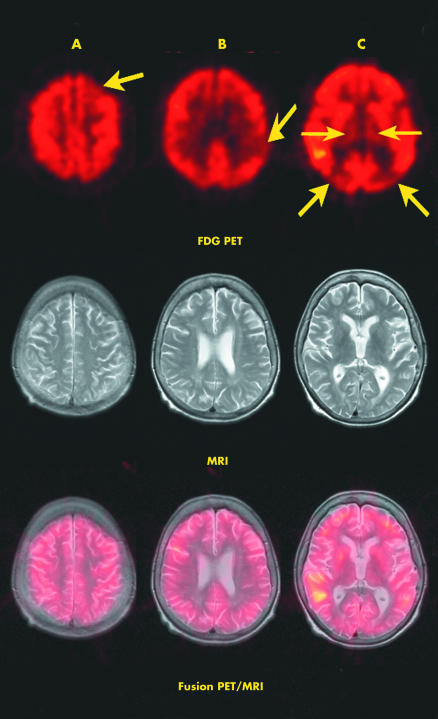
The NPC1 gene, mapped to chromosome 18q11–q12, spans 56 kb and contains 25 exons. It encodes a 1278‐amino‐acid integral membrane protein with 13 transmembrane domains.1,2 Molecular genetic analysis showed a new frameshift mutation of the NPC1 gene, K1206fs, on one allele in our patient. By cDNA sequencing, the second allele remained so far unidentified, a not uncommon situation for patients with NPC1.1 Neuroimaging of fluorodeoxyglucose (FDG)‐PET labelled with fluorine‐18 showed remarkable hypometabolism bilateral in thalamic and parietooccipital cortical regions (fig 1), in good agreement with the recent observation that diminished thalamic metabolism is a shared feature on FDG‐PET in patients with NPC.3
Figure 1 Image fusion of transversal slices each of positron emission tomography (PET) and T2‐weighted magnetic resonance imaging (MRI). Decreased uptake of 18F fluorodeoxy‐glucose (18F FDG; upper row) in the left frontal cortex (A, arrow), left parietal cortex (B, arrow), left and right parietooccipital cortex (C, thick arrows) and both thalami (C, thin arrows).
After discontinuation of drug treatment for neuroleptic and Parkinson's disease, the cognitive functions of the patient improved without relevant drugs. Interestingly, during a period of 2 weeks, the patient communicated only in English, a language spoken temporarily during early primary school. Finally, she was able to eat and to communicate in a simplified fashion. At present, 4 years later, the patient lives in a nursing home and still displays fluctuating daily abilities.
Conclusion
NPC represents an autosomal recessive disorder with a major biochemical, molecular and clinical variability. We report on the oldest patient with NPC, which highlights this important, although rare, diagnosis of which neurologists and psychiatrists need to be aware and which, hopefully, might be treated in the near future. Ongoing research suggests that substrate reduction treatment may be able to halt or slow progression of the disease, particularly in milder cases such as the one presented.4 At present, clinical trials with an inhibitor of glycosphingolipid biosynthesis (miglustat) are under way in patients with NPC.5
To enable such treatment, however, the organic cause also has to be identified early in patients with psychiatric presentations. Thus, there is need for a thorough physical and biochemical assessment in such patients to avoid missing these sorts of diagnosis.
References
- 1.Vanier M T, Millat G. Niemann‐Pick disease type C. Clin Genet 200364269–281. [DOI] [PubMed] [Google Scholar]
- 2.Patterson M C. A riddle wrapped in a mystery: understanding Niemann‐Pick disease, type C. Neurologist 20039301–310. [DOI] [PubMed] [Google Scholar]
- 3.Klünemann H H, Ibach B, Guerlach G.et al Neurologic, psychiatric and neuropsychologic evaluation combined with F‐18‐fluorodeoxyglucose (FDG)‐PET neuroimaging in adult patients with Niemann‐Pick disease type C. Ann Neurol 200150S61 [Google Scholar]
- 4.Lachmann R H, te Vruchte D, Lloyd‐Evans E.et al Treatment with miglustat reverses the lipid‐trafficking defect in Niemann‐Pick disease type C. Neurobiol Dis 200416654–658. [DOI] [PubMed] [Google Scholar]
- 5.Cox T M. Substrate reduction therapy for lysosomal storage diseases. Acta Paediatr Suppl 20059469–75. [DOI] [PubMed] [Google Scholar]