Abstract
Background
Mutations of myelin protein zero (MPZ) may cause inherited neuropathy with variable expression.
Objective
To report phenotypic variability in a large American kindred with MPZ mutation His39Pro.
Patients
Genetic testing was performed on 77 family members and 200 controls. Clinical and electrophysiological field study assessments were available for review in 47 family members.
Results
His39Pro was found in all 10 individuals prospectively identified with neuropathy. 200 normal controls were without mutation. Symptoms of neuropathy began in adulthood and were slowly progressive except for one acute-onset painful sensory neuropathy. Associated features included premature hearing loss (n = 7), nocturnal restless leg symptoms (n = 8) and multiple sclerosis in one.
Conclusions
MPZ mutation His39Pro may be associated with acute-onset neuropathy, early‐onset hearing loss and restless legs. The relationship with multiple sclerosis in the proband remains uncertain.
Among inherited neuropathies, attempts at correlating phenotype with genotype have met with variable success. Success depends on the specific gene, number of patients studied and the methods by which clinical involvement was ascertained. Of the different genetic causes of hereditary motor and sensory neuropathies, myelin protein zero (MPZ) mutations account for considerable variability, with more than 90 mutations described.1 Variable degrees of hearing loss,2,3 pupillary areflexia to light and accommodation,3 and diaphragmatic weakness4 or chronic cough5 have been reported. Frequent association with restless‐leg‐like symptoms has not been established. The clinical and electrophysiological variability in patients with MPZ mutations may be seen even among monozygotic twins.6 Previous studies on families with MPZ mutations were usually carried out on a small number of people. In the kinship reported here, many family members and other controls were studied.
On the basis of multiple lines of evidence, changes in immunity represent an attractive potential modifying mechanism for the varied presentations of MPZ mutations.7,8,9 Rare mutations of the peripheral myelin proteins (PMP22) and connexin 32 have been associated with changes in the central nervous system (CNS) on imaging, characteristic of multiple sclerosis and acute disseminated encephalomyelitis, respectively.10,11,12,13,14 In this report, we present data on a large family with the MPZ mutation His39Pro and describe the varied phenotypes seen in the kindred, including one case of acute‐onset painful neuropathy, hearing loss, restless legs and relapsing remitting multiple sclerosis.
Methods
Proband evaluation
At age 27 years, the proband (III‐40) developed right hemiparesis over 4 days, which resolved after 6 weeks. Symptoms of neuropathy appeared at 37 years, with a gradual symmetrical distal sensory loss and nocturnal “fidgety feet”. Electrophysiological abnormalities consistent with a mixed axonal and demyelinating peripheral neuropathy were documented. Between the ages of 41 and 51 years, the patient had several relapses of asymmetric incoordination or weakness.
Serial magnetic resonance images showed multiple small bilateral white matter lesions that increased in number and enhanced with episodes (fig 1D). Analysis of the cerebrospinal fluid (CSF) showed three oligoclonal bands and an increased rate of intrathecal immunoglobulin G (IgG) synthesis. Extensive laboratory testing for infectious, inflammatory or metabolic causes did not yield positive results. The McDonald criteria for multiple sclerosis were met.
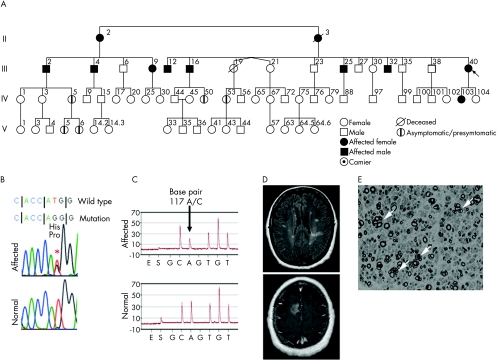
Figure 1 (A) Large American kindred of Northern European descent affected by peripheral neuropathy, hearing loss, restless legs and relapsing remitting multiple sclerosis in the proband with myelin protein zero (MPZ) missense mutation His39Pro. People designated as affected were determined to have neuropathy before genetic testing confirmed the mutation. Genetic testing was carried out on all people shown, except for III‐19, who died in a motor vehicle accident at age 36 years without known symptoms and II‐3, who, family members believed, was definitely affected by neuropathy. One person IV‐53 at age 37 years was identified to have the mutation, but had no symptoms, signs or electrophysiological evidence for neuropathy. Four young descendants (IV‐5, IV‐50, V‐5 and V‐6; ages 28, 24, 5 and 2 years, respectively) of affected people were positive for genetic testing and declined examination. Hearing loss and restless legs were common among those affected by neuropathy (table 1). (B) The reverse complimentary missense mutation His39Pro, 117TG (His39Pro) in MPZ. (C) Confirmatory forward‐strand pyrosequencing analysis of the mutation (above) and normal (below), showing the heterozygotic A and C base‐pair population at position 117. Each peak (y axis) represents the luminescent reaction with successful incorporation of the specific purine or pyrimidine base released for incorporation (x axis). (D) Magnetic resonance image (fluid‐attenuated inversion recovery and gadolinium‐enhanced sequences) of the proband (III‐40) at age 57 years, showing multiple periventricular T2‐weighted hyperintense white matter lesions and a contrast‐enhancing lesion of the right centrum semiovale, consistent with multiple sclerosis. (E) Semithin sections stained with methylene blue from sural nerve biopsy of the proband, showing marked reduction in the number of large myelinated fibres and numerous regenerating clusters (arrows).
The sural nerve biopsy results showed a marked reduction in the number of large myelinated fibres and many regenerating clusters (fig 1E). Commercially available tests for connexin 32, early growth response 2, neurofilament—light and PMP22 mutations were negative. MPZ sequencing showed an A→C substitution at nucleotide position 117.
Kindred
In all, 47 available members of the family were evaluated by neurological examination and nerve conduction studies. A total of 77 family members were subjected to DNA testing for mutations of the MPZ gene. Mutation analysis was carried out by conventional means, with confirmatory pyrosequencing (fig 1B,C). A DNA library of normal controls of Northern European descent15 was available.
Results
Clinical features
Ten people were prospectively diagnosed with neuropathy (table 1). The neuropathy in these 10 people had the following features: slowly progressive length‐dependent sensorimotor symptomatic neuropathy (n = 5); asymptomatic electrophysiological abnormalities with neuropathic signs (n = 4); and acute‐onset neuropathic pain and new neuropathic signs (n = 1). Nocturnal akathisia of the feet and legs, relieved by pacing characteristic of restless legs syndrome, was elicited by telephone interview (n = 8). Progressive sensorineural hearing loss was recorded (n = 7). Hearing aids (n = 3) were required by the age of 40 years.
Table 1 Clinical and electrophysiological* features in people with genetically confirmed neuropathy.
| Pedigree | Age (years) | Sex | Clinical features | RLS | Age at hearing loss (years) | Median motor | Peroneal motor | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset | Aids | Amp (mV) | CV (m/s) | Amp (mV) | CV (m/s) | Sural Amp (μV) | |||||
| II‐02 | 78 | F | Asymptomatic pes cavus, distal weakness, areflexia | Yes | 58 | 68 | 3.8 | 49 | 2.2 | 38 | 0 |
| III‐02 | 60 | M | Asymptomatic distal weakness and sensory loss | Yes | 50 | 57 | 5.5 | 39 | 0.3 | 34 | 0 |
| III‐04 | 57 | M | Asymptomatic distal weakness and sensory loss | Yes | 18 | 50 | 3.4 | 33 | 0 | NA | 0 |
| III‐09 | 52 | F | Lower limb areflexia | Yes | 50 | No | 7 | 42 | 0.3 | 37 | 0 |
| III‐12 | 58 | M | Distal paraesthesiae | Yes | <58 | No | 5 | 38 | 1.7 | 39 | 0 |
| III‐16 | 56 | M | Bilateral foot drop and distal dysaesthesia | Yes | 40 | 40 | 0.3 | 28 | 0.2 | 26 | 0 |
| III‐25 | 50 | M | Distal paraesthesiae, distal areflexia | No | 18 | 28 | 1.5 | 38 | 1.9 | 40 | 0 |
| III‐32 | 45 | M | Distal hypoaesthesia | Yes | 35 | 35 | 10.5 | 48 | 1.6 | 39 | 0 |
| III‐40 | 51 | F | Mild distal weakness and distal hypoaesthesia | Yes | 51 | No | – | – | 1.1 | 38 | 0 |
| IV‐103 | 30 | F | Acute‐onset severe acral burning pain | No | No | NA | – | – | 4.4 | 30 | 3 |
Amp, amplitude; CV, conduction velocity; NA, not applicable; RLS, restless legs syndrome.
Abnormal values are bold type. Abnormal conduction block or temporal dispersion was not seen.
A patient with acute onset
Family member IV‐103 was initially assessed and found to have no abnormality (including neurological examination and nerve conduction values). At 30 years of age, she awoke with severe burning and shock‐like pain more in the distal feet than in the hands. Neurological examination showed absent or severely reduced tendon reflexes. Vibration and proprioception were reduced at the toes, whereas pin and hot anvil testing (40°C) showed hyperalgesia in a symmetrical glove‐and‐stocking distribution. Pupillary reflexes were normal.
Nerve conduction studies and electromyography carried out 1 week after the onset of symptoms showed reduced sural, median and ulnar sensory potential amplitudes. Magnetic resonance images (including gadolinium‐enhanced sequences) of the head and spinal cord were normal. The protein level in the CSF was 59 mg/dl (laboratory normal, 14–45 mg/dl). A sural nerve biopsy specimen taken 2 weeks after the onset of symptoms showed normal myelinated fibre density and no inflammatory infiltration. Teased fibres showed increased rates of segmental demyelination (6%; upper limit of normal, 2%) and myelin reduplication (14%; upper limit of normal, 7%).
With an aggressive plan for pain management and rehabilitation, symptoms improved, whereas nerve conduction findings remained unchanged.
Genetic testing
The His39Pro mutation segregated with all 10 affected members of the family, whereas among people assessed as unaffected by neuropathy and in whom genetic testing was carried out (25 family members), one person had the mutation. Of an additional 42 family members who were not clinically assessed, the mutation was found in four. By telephone interview, these people (aged 17–42 years) had no symptoms. The mutation was not found in the 200 healthy controls.
Discussion
The inherited neuropathy studied here is transmitted as a dominant trait with variable expression, begins in the third to fifth decades of life, may begin with restless legs or acute pain, and is often associated with hearing loss. Although the occurrence of multiple sclerosis in the proband may be related to chance, a similar association with the MPZ mutation Thr124Met has recently been reported.16 Intrafamilial phenotypic variability with MPZ mutations is well recognised,17,18 and in this kindred included variable age and mode of onset and the severity and pattern of clinical and electrophysiological abnormalities.
We attribute the neuropathy and other possible clinical features to the extracellular His39Pro mutation for the following reasons:
All members prospectively identified as affected by neuropathy had the mutation.
The mutation was not found in 200 healthy controls without neuropathy.
The prediction of a changed gene product amino acid sequence.
Four patients affected by isolated neuropathy, with mutation testing identifying His39Pro19 (reported as His10Pro, ie, without signal peptide counted), were previously independently reported.
The mutation (His39Pro) is a histidine→proline missense change that occurs in the extracellular MPZ domain and is predicted to secondarily change the protein structure by introducing a non‐polar neutral amino acid from a polar charge. Further studies are required to predict whether this mutation interferes with adhesion properties of the molecule or compact myelin formation, or produces changed immunogenicity. It is an attractive possibility that inflammation or changed immunity may be a consequence of this mutation and account for some of the observed varied expression. Such a hypothesis may be supported by our patient with an acute‐onset disease, as this temporal profile is typical of an inflammatory neuropathy. With this thought in mind, we searched for evidence of inflammation in the sural nerve biopsy specimen that was obtained in 2 weeks of the onset of symptoms, but found no such evidence. Nevertheless, our studies do not exclude more proximal inflammation that would go unrecognised in a distal nerve biopsy. Consideration of that possibility is emphasised by prior work on immune mechanisms in MPZ pathogenesis: (1) chronic inflammatory demyelinating polyneuropathy‐like polyneuropathy partially responsive to steroids has been described in patients with MPZ mutations8; (2) heterozygous knockout mice (Mpz+/Mpz−) develop clinical and histopathological features similar to chronic inflammatory demyelinating polyneuropathy9; and (3) Mpz+/Mpz− mice that are genetically unable to generate an immune response develop a less severe polyneuropathy than their immune‐competent controls.7
Hearing loss and pupillary reflex abnormalities have been described in families with late‐onset motor and sensory neuropathy‐2 associated with the Thr124Met MPZ mutation.3,20 Hearing loss without pupillary abnormalities presenting years before the onset of neuropathy has also been reported in association with a Glu97Val mutation.2 An early‐onset and sometimes a severe sensorineural hearing loss was a feature of our kindred. These mutations are all located in the extracellular domain.
Restless legs syndrome is a common disorder with an estimated population prevalence of 11%,21 and has been reported in as many as 37% of unselected people with a Charcot–Marie–Tooth type‐II phenotype.22 Symptoms consistent with restless legs syndrome occurred in 8 of the 10 affected people in our kindred and typically preceded the symptomatic onset of neuropathy by years.
Multiple sclerosis or subclinical demyelination of the CNS is a recognised association of chronic inflammatory demyelinating polyradiculoneuropathy.23 More rarely, multiple sclerosis occurs in association with inherited demyelinating neuropathies with PMP22 duplication12,13 or deletion.10,14 MPZ is not expressed in the CNS24 and it is possible that the association of His39Pro MPZ mutation with multiple sclerosis in our proband is due to chance. But, in conjunction with the recent report on a patient with MPZ, extracellular domain mutation Thr124Met and immunotherapy‐responsive relapse‐remitting multiple sclerosis, the possibility of a causal association should be considered and other reports are sought. The pathogenic mechanism of such a potential association is uncertain, but a change in the antigenic potential of the extracellular protein with a conserved epitope in the brain may be postulated. Migration of peripheral Schwann cells into the spinal cord after traumatic injury has been documented,25 and represents a potential mechanism whereby MPZ antigens may be presented to the CNS.
Phenotypic variability with the MPZ mutation is emphasised by this study on a large kindred. Clinicians should consider acute‐onset neuropathy, hearing loss and restless legs in the decision on MPZ sequencing.
Abbreviations
CSF - cerebrospinal fluid
MPZ - myelin protein zero
PMP - peripheral myelin protein
Footnotes
Funding: This study was supported in part by a grant obtained from the National Institute of Neurological Disease and Stroke (NINDS 36797).
Competing interests: PJD is one of the inventors of the CASE IV system (manufactured and marketed by WR Medical Electronics, Stillwater, Minnesota, USA), with test results from use of the prototype systems developed by us reported here. Royalties from this intellectual property did not exceed the federal threshold of $10 000 per year and was given to charity.
References
- 1.Shy M E. Hereditary motor and sensory neuropathies related to MPZ (P0) mutations, in Peripheral Neuropathy. In: Dyck PJT, PK , eds. Philadelphia: Elsevier Saunders, 20051681–1706.
- 2.Seeman P, Mazanec R, Huehne K.et al Hearing loss as the first feature of late‐onset axonal CMT disease due to a novel P0 mutation. Neurology 200463(4)733–735. [DOI] [PubMed] [Google Scholar]
- 3.De Jonghe P, Timmerman V, Ceuterick C.et al The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot‐Marie‐Tooth phenotype. Brain 1999122(Pt 2)281–290. [DOI] [PubMed] [Google Scholar]
- 4.Stojkovic T, de Seze J, Dubourg O.et al Autonomic and respiratory dysfunction in Charcot‐Marie‐Tooth disease due to Thr124Met mutation in the myelin protein zero gene. Clin Neurophysiol 2003114(9)1609–1614. [DOI] [PubMed] [Google Scholar]
- 5.Baloh R H, Jen J C, Kim G.et al Chronic cough due to Thr124Met mutation in the peripheral myelin protein zero (MPZ gene). Neurology 200462(10)1905–1906. [DOI] [PubMed] [Google Scholar]
- 6.Marques W, Jr, Hanna M G, Marques S R.et al Phenotypic variation of a new P0 mutation in genetically identical twins. J Neurol 1999246(7)596–599. [DOI] [PubMed] [Google Scholar]
- 7.Maurer M, Schmid C D, Bootz F.et al Bone marrow transfer from wild‐type mice reverts the beneficial effect of genetically mediated immune deficiency in myelin mutants. Mol Cell Neurosci 200117(6)1094–1101. [DOI] [PubMed] [Google Scholar]
- 8.Donaghy M, Sisodiya S M, Kennett R.et al Steroid responsive polyneuropathy in a family with a novel myelin protein zero mutation. J Neurol Neurosurg Psychiatry 200069(6)799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shy M E, Arroyo E, Sladky J.et al Heterozygous P0 knockout mice develop a peripheral neuropathy that resembles chronic inflammatory demyelinating polyneuropathy (CIDP). J Neuropathol Exp Neurol 199756(7)811–821. [PubMed] [Google Scholar]
- 10.Sanahuja J, Franco E, Rojas‐Garcia R.et al Central nervous system involvement in hereditary neuropathy with liability to pressure palsies: description of a large family with this association. Arch Neurol 200562(12)1911–1914. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R A, Simon E M, Marks H G.et al The CNS phenotype of X‐linked Charcot‐Marie‐Tooth disease: more than a peripheral problem. Neurology 200361(11)1475–1478. [DOI] [PubMed] [Google Scholar]
- 12.Almsaddi M, Bertorini T E, Seltzer W K. Demyelinating neuropathy in a patient with multiple sclerosis and genotypical HMSN‐1. Neuromuscul Disord 19988(2)87–89. [DOI] [PubMed] [Google Scholar]
- 13.Frasson E, Polo A, Di Summa A.et al Multiple sclerosis associated with duplicated CMT1A: a report of two cases. J Neurol Neurosurg Psychiatry 199763(3)413–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato A A, Barohn R J. Hereditary neuropathy with liability to pressure palsies: assocation with central nervous system demyelination. Muscle Nerve 199619(6)770–773. [DOI] [PubMed] [Google Scholar]
- 15.Dyck P J, Litchy W J, Lehman K A.et al Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of Healthy Subjects. Neurology 199545(6)1115–1121. [DOI] [PubMed] [Google Scholar]
- 16.Rajabally Y A, Abbott R J. Charcot‐Marie‐Tooth disease due to the Thr124Met mutation in the myelin protein zero gene associated with multiple sclerosis. J Peripher Nerv Syst 200510(4)388–389. [DOI] [PubMed] [Google Scholar]
- 17.Szabo A, Suchner S, Siska E.et al Marked phenotypic variation in a family with a new myelin protein zero mutation. Neuromuscul Disord 200515(11)760–763. [DOI] [PubMed] [Google Scholar]
- 18.Sowden J E, Logigian E L, Malik K.et al Genotype‐phenotype correlation in a family with late onset CMT and an MPZ lys236del mutation. J Neurol Neurosurg Psychiatry 200576(3)442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shy M E, Jani A, Krajewski K.et al Phenotypic clustering in MPZ mutations. Brain 2004127(Pt 2)371–384. [DOI] [PubMed] [Google Scholar]
- 20.Chapon F, Latour P, Dirason P.et al Axonal phenotype of Charcot‐Marie‐Tooth disease associated with a mutation in the myelin protein zero gene. J Neurol Neurosurg Psychiatry 199966(6)779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hening W, Walters A S, Allen R P.et al Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med 20045(3)237–246. [DOI] [PubMed] [Google Scholar]
- 22.Gemignani F, Marbini A, Di Giovanni G.et al Charcot‐Marie‐Tooth disease type 2 with restless legs syndrome. Neurology 199952(5)1064–1066. [DOI] [PubMed] [Google Scholar]
- 23.Mendell J R, Kolkin S, Kissel J T.et al Evidence for central nervous system demyelination in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 198737(8)1291–1294. [DOI] [PubMed] [Google Scholar]
- 24.Brockes J P, Raff M C, Nishiguchi D J.et al Studies on cultured rat Schwann cells. III. Assays for peripheral myelin proteins. J Neurocytol 19809(1)67–77. [DOI] [PubMed] [Google Scholar]
- 25.Guest J D, Hiester E D, Bunge R P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 2005192(2)384–393. [DOI] [PubMed] [Google Scholar]