Abstract
Objective
To assess overall resource consumption, work capacity and quality of life of patients with multiple sclerosis in nine European countries.
Methods
Information on resource consumption related to multiple sclerosis, informal care by relatives, productivity losses and overall quality of life (utility) was collected with a standardised pre‐tested questionnaire from 13 186 patients enrolled in national multiple sclerosis societies or followed up in neurology clinics. Information on disease included disease duration, self‐assessed disease severity and relapses. Mean annual costs per patient (€, 2005) were estimated from the societal perspective.
Results
The mean age ranged from 45.1 to 53.4 years, and all levels of disease severity were represented. Between 16% and 29% of patients reported experiencing a relapse in the 3 months preceding data collection. The proportion of patients in early retirement because of multiple sclerosis ranged from 33% to 45%. The use of direct medical resources (eg, hospitalisation, consultations and drugs) varied considerably across countries, whereas the use of non‐medical resources (eg, walking sticks, wheel chairs, modifications to house and car) and services (eg, home care and transportation) was comparable. Informal care use was highly correlated with disease severity, but was further influenced by healthcare systems and family structure. All types of costs increased with worsening disease. The total mean annual costs per patient (adjusted for gross domestic product purchasing power) were estimated at €18 000 for mild disease (Expanded Disability Status Scale (EDSS) <4.0), €36 500 for moderate disease (EDSS 4.0–6.5) and €62 000 for severe disease (EDSS >7.0). Utility was similar across countries at around 0.70 for a patient with an EDSS of 2.0 and around 0.45 for a patient with an EDSS of 6.5. Intangible costs were estimated at around €13 000 per patient.
The treatment of patients with multiple sclerosis has changed over the past 10 years, with several new potent treatments introduced in an area where treatment options had been limited. Compared with the old and inexpensive symptomatic treatments, the new disease‐modifying drugs (DMDs) seem costly, and it must be expected that healthcare costs for patients with multiple sclerosis have increased. Also, the new treatments are likely to lead to more intensive patient management, thus potentially increasing costs further. Finally, as our knowledge of multiple sclerosis has improved, pathological and therapeutic criteria have been modified and a diagnosis is often made earlier, increasing the patient population that is eligible for treatment and thus potentially increasing treatment costs. As a consequence, the interest in economic evaluation of multiple sclerosis has intensified.
The relevant economic question today is whether investment in more costly treatments is a good use of scarce resources. Evidence of the cost effectiveness of new treatments must be shown for them to be adopted and paid for by healthcare services. Cost‐effectiveness analysis in multiple sclerosis is, however, not straightforward. Treatment for multiple sclerosis aims at avoiding temporary disability due to relapses and, more importantly, delaying the progression to more permanent disability. Thus, the major economic benefit of treatment lies in the future; savings will come from delaying or preventing patients' progression to more severe disease, which is associated with high costs and low quality of life (QoL). Clinical trials are, however, too short to provide full evidence of the benefits of DMDs, and modelling has become the accepted standard for economic evaluation in multiple sclerosis.
Cost‐effectiveness models in multiple sclerosis combine epidemiological data on the natural history of the disease, consumption of healthcare and other resources, work capacity over the entire course of the disease, QoL related to disease severity and data on the effectiveness of treatments to change the disease course.1,2,3,4,5,6,7,8,9
Current data on the overall burden of multiple sclerosis in Europe are scarce. Several cost studies were carried out in the early and mid‐1990s,10,11,12,13,14,15,16 when DMDs were not yet established as part of standard treatment. Thus, the findings of these studies may no longer be accurate. Other studies have focused on patients treated with DMDs,17,18 and findings are therefore not representative of the total patient population.
The objective of this Europe‐wide observational study was to establish the current cost of multiple sclerosis at different levels of disease severity, to provide a basis on which the economic effect of new treatments can be estimated. It is possible to combine this information with reliable data on the prevalence of multiple sclerosis, including prevalence at different levels of severity, to estimate the total cost of multiple sclerosis in a given country or geographical area.
Patients and methods
This study is based on the method used in several earlier studies on multiple sclerosis in Europe and the US.15,16,19,20 Information on demographics, resource utilisation, work capacity and QoL was collected in a cross‐sectional anonymous mail survey. The study enrolled patients at all levels of disease severity to permit estimation of the effect of disease progression on costs and QoL.
Participants
The study was conducted in nine European countries, in collaboration with neurology clinics and national multiple sclerosis societies. In six countries, the questionnaire was mailed by the multiple sclerosis societies to their members (Austria, Italy, Spain, Sweden, Switzerland and the UK). In the three remaining countries (Belgium, Germany and The Netherlands), multiple sclerosis societies had other ongoing surveys at the time of this study and patients were therefore enrolled solely through neurology clinics, with appropriate ethical approvals.
The questionnaire informed patients about the purpose of the study and about how data would be used. Patients gave written consent to use the information they provided for research and publication.
Data
The questionnaire asked for background information on age, sex, marital status, living situation, education level, employment status and QoL. Disease information was limited to age at onset of first symptoms of multiple sclerosis, year of diagnosis, type of multiple sclerosis, exacerbations during the past 3 months and a self‐assessment of disability. A description of disease severity was developed and the scale was tested on a small sample of patients before inclusion in the questionnaire. This scale focused on ambulation and was based on the original description in the Expanded Disability Status Scale (EDSS)21 and on the Patient Determined Disease Steps instrument.22
The objective of the study was to determine the cost of multiple sclerosis, as opposed to the cost of a patient with multiple sclerosis. Patients were therefore asked to include only consumption related to multiple sclerosis. Different recall periods were used for different resources, on the basis of experience in previous studies. Questions on inpatient admissions, consultations, investigations and short‐term absences from work related to the past 3 months; those on drugs and services such as home care, informal care from family and friends related only to the past month; and questions on major investments such as wheel chairs, scooters or transformations to the house and car related to the past year. In addition, working patients were asked to indicate whether they had to change their type of work or working hours because of multiple sclerosis, and patients who were on early retirement were asked to confirm that it was because of multiple sclerosis.
Data on QoL were collected as utility with a generic preference‐based instrument, the EuroQol (EQ‐5D).23 The EQ‐5D covers five domains of health‐related QoL (mobility, self‐care, usual activities, pain or discomfort, and anxiety or depression), with three levels of answers (no, some and severe problems). The resulting combination of answers can then be translated into utilities via a social tariff established with the general population by using decision–analytical methods (time–trade‐off).24 Although reference scores for the EQ‐5D are available in many countries, the tariff for the UK is the only one in Europe, which is based on decision–analytical methods (time–trade‐off),25 and has been widely used, including in multiple sclerosis. To compare results across countries and with earlier studies, it was used for all countries in this study.
Data management and analysis
Completed questionnaires were entered on to an online database that included both numerical and logical checks to minimise errors. Before analysis, missing answers and outliers were systematically verified. In a small number of situations—for example, when a patient had indicated using a resource, but had omitted the quantity, the mean quantities for users of the same resource were imputed. For some items—for example, investments, patients were asked to indicate the cost because no standard unit costs were available. To avoid problems with potential outliers, we assumed that no such costs would be higher than the sample mean+1SD.
Unit costs were obtained from several publicly available sources and telephone interviews and, if necessary, adjusted to 2005 prices with the consumer price index. Utilisation was annualised and the main analysis was carried out from the societal perspective, using opportunity costs regardless of payment. In countries with social insurance systems, costs are also presented from the perspective of the payer (healthcare and social services).
Hospitalisation costs were generally based on admissions using diagnosis‐related groups, whereas costs of inpatient stays in nursing homes or rehabilitation centres were based on costs per day. Consultation costs were based on cost per visit, whereas costs of sessions with healthcare professionals such as physiotherapists were estimated on the average duration of a session. The cost of drugs was estimated from average public prices across pack sizes, dosage strengths and recommended doses. Where generics were available, the prices of branded and generic products were weighted according to the estimated generics penetration in each country. Specific drugs for multiple sclerosis were generally assumed to be self‐injected (except for a small proportion of patients using once‐a‐week intramuscular interferon beta (IFNβ)‐1a, where injections were given by a nurse). Costs of over‐the‐counter drugs and investments were based directly on patients' indications.
Production losses were valued with the human capital approach, where the production of a person is valued at the market price (in this case, the sex‐specific average salary including employers' costs). For short‐term sick leave, labour costs were adjusted to patients' working hours, whereas for long‐term sick leave and early retirement due to multiple sclerosis, the national average annual working time by sex was used. This method of estimation of indirect costs is the most commonly used one in economic studies, although it has been suggested that it may overestimate costs, as, particularly in times of unemployment, a worker would be rapidly replaced and hence no production loss would occur. A different method of calculation (friction cost method26) has therefore been proposed, but is not generally used.
Informal care was valued using the net disposable income after taxes. Other methods to estimate informal care include the replacement method, where the care would be provided by a professional rather than a family member, or production losses for family members who are working. The first method may lead to an overestimation, as not all informal help would be provided by a professional, whereas the second assigns no value to time spent by non‐working family members.
The mean cost of a relapse in multiple sclerosis was calculated as the difference in costs during the preceding 3 months between patients with a relapse and those without. Cost calculations were based on patients who had an EDSS <5.0, as relapses are more common in early disease and may have a larger effect on patients with limited permanent disability.
Finally, intangible costs—that is, costs due to pain, grief, anxiety and social handicap, are also estimated. These costs are generally omitted in cost‐of‐illness studies, whereas in cost‐effectiveness analyses they are included in the outcome assessment. We calculated the loss of quality‐adjusted life years (QALYs) from the difference in utility scores between patients with multiple sclerosis, matched for sex and age, in the samples and in the general population in the respective country. By assigning a monetary value to a QALY—for example, €50 000 or three times the gross domestic product (GDP) per capita,27 intangible costs can be calculated.
Results
Patients
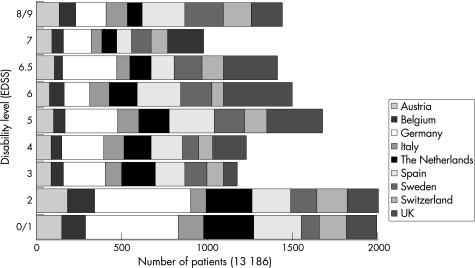
The study included a total of 13 186 patients at all levels of disability (fig 1). Owing to the survey method used and the difficulty of completing an extensive questionnaire, however, the number of patients with very severe disability (EDSS 9.0–9.5) was somewhat limited at just over 1% of the sample, and the prevalence of these patients may be underestimated.
Figure 1 Patients at all levels of disease severity (Expanded Disability Status Scale (EDSS)). Patients with limited disability were over‐represented in the German sample where, in addition to patients in specialised clinics, a panel of patients with early disease who had earlier expressed an interest in participating in surveys was included.
Table 1 shows the main characteristics of the samples. The sample size in the different countries ranged from 799 to 2793 patients, with response rates varying between 19% and 75%. Patients recruited in neurology clinics tended to have a shorter disease duration and less permanent disability (lower mean EDSS) than those recruited by patient associations. By contrast, patients recruited by neurology clinics had a higher number of relapses. This was expected, because such clinics are likely to follow up patients with active disease.
Table 1 Characteristics of the sample.
| Austria | Belgium | Germany | Italy | The Netherlands | Spain | Sweden | Switzerland | UK | |
|---|---|---|---|---|---|---|---|---|---|
| Recruitment | NMSS | Clinics | Clinics | NMSS | Clinics | NMSS | NMSS | NMSS | NMSS |
| Response rate (%) | 35 | 38 | 38 | 31 | 52 | 32 | 75 | 45 | 19 |
| Sample size (n) | 1019 | 799 | 2793 | 921 | 1549 | 1848 | 1339 | 1101 | 2048 |
| Women (%) | 70.4 | 68.0 | 72.2 | 65.8 | 69.1 | 64.2 | 73.0 | 63.8 | 74.5 |
| Living alone (%) | 28.1 | 18.8 | 21.1 | 12.5 | 15.5 | 9.7 | 27.9 | 22.6 | 14.3 |
| Mean (SD) age (years) | 50.0 (12.2) | 48.1 (12.6) | 45.1 (11.1) | 46.1 | 46.7 (11.1) | 44.7 (10.8) | 53.4 (12.0) | 52.5 (12.8) | 51.4 (10.7) |
| Aged >65 years (%) | 13.0 | 12.4 | 17.7 | 8.5 | 7.6 | 5.5 | 16.1 | 21.4 | 10.3 |
| Employed and working (%) | 29.8 | 33.9 | 38.6 | 41.5 | 35.5 | 26.2 | 34.1 | 33.5 | 27.3 |
| Employed but on long‐term sick leave (%) | 0.6 | 5.8 | 2.3 | 0.6 | 1.9 | 3.8 | 6.7 | 1.2 | 0.9 |
| Early retirement due to MS | 44.5 | 32.9 | 33.9 | 33.3 | 42.2 | 34.1 | 35.6 | 33.9 | 44.3 |
| Mean (SD) age at diagnosis (years) | 34.7 (10.4) | 35.3 (10.8) | 35.0 (10.2) | 33.8 (10.3) | 37.0 (10.2) | 33.0 (9.9) | 39.3 (10.7) | 36.2 (10.6) | 38.8 (10.0) |
| Mean (SD) age at first symptoms (years) | 31.5 (10.2) | 31.8 (10.7) | 31.8 (10.0) | 29.9 (10.0) | 31.2 (9.9) | 29.5 (10.0) | 32.0 (10.7) | 33.4 (10.9) | 32.2 (10.4) |
| Mean (SD) EDSS | 4.4 (2.4) | 4.2 (2.4) | 3.8 (2.3) | 4.6 (2.3) | 3.9 (2.2) | 4.5 (2.3) | 5.1 (2.2) | 4.5 (2.4) | 5.1 (2.0) |
| Relapse in past 3 months (%) | 16.8 | 21.5 | 24.4 | 21.5 | 29.2 | 22.6 | 18.0 | 16.3 | 28.9 |
| Unsure regarding relapse or missing (%) | 13.0 | 16.1 | 16.3 | 29.3 | 27.4 | 17.2 | 26.7 | 14.5 | – |
Clinics, neurology clinics; MS, multiple sclerosis; NMSS, national multiple sclerosis societies (patient associations).
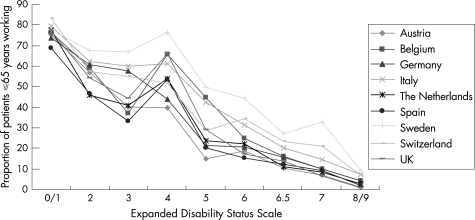
The proportion of patients who were working ranged between 25% and 40%, depending to some extent on the proportion of patients aged >65 years in the samples. No other obvious relationship with disease status of the sample was evident, however. Similarly, although on average 35% of patients were in early retirement because of multiple sclerosis, the proportion was considerably higher (around 45%) in Austria, The Netherlands and the UK. The reasons for this were not obvious. These results probably reflect differences in general workforce participation and handling of long‐term sick leave and invalidity pensions in different countries rather than differences in the samples or disease severity. The effect of the disease on employment is, however, very pronounced (fig 2). Although at EDSS 0.0–1.0, about 70–80% of patients <65 years are employed, this proportion is <10% for patients with EDSS 8.0–9.0. In addition, several employed patients were on long‐term sick leave at the time of the survey, reducing the number of patients actually able to work. Lastly, >50% of patients indicated that they had to reduce the number of hours worked or change their type of work. Most often, this was associated with a loss of income (not included in this analysis).
Figure 2 The proportion of patients employed or on long‐term sick leave is calculated as a percentage of patients aged ⩽65 years. The reduced work capacity particularly at Expanded Disability Status Scale (EDSS) 2–3 compared with EDSS 4 is explained by an increased frequency of relapses in this group and hence a concentration of sick leave. A similar finding can be seen in fig 6, where utility at EDSS 3 is relatively low.
Resource use
The use of direct medical resources is often influenced by the disease and the organisation of the healthcare system, medical tradition, ease of access and availability. This becomes evident when comparing consumption across the countries. Despite the relative similarity in the samples, inpatient admissions and length of stay, medical consultations, physiotherapy and the use of DMDs show substantial variation (table 2). Similarly, although the proportions of patients who use services such as home help or who make investments such as modifications to the house or the car are more comparable across the countries than the proportions of patients using other healthcare resources, their availability or their cost none the less influences consumption. Thus, considerable differences exist in the intensity of the usage of services, as different countries provide different levels of service. A good example is Sweden, where patients with severe diseases are offered extensive support through personal assistants employed by the healthcare system. Other countries seem to provide limited support and patients therefore require substantial help from friends and families (eg, in Italy or in the UK). Also, informal care is influenced by tradition and family structures. In countries where the proportion of women at home is larger, the use of informal care may be higher because of both availability and the type of activities that are reported as informal care by respondents.
Table 2 Consumption of resources.
| Austria | Belgium | Germany | Italy | The Netherlands | Spain | Sweden | Switzerland | UK | |
|---|---|---|---|---|---|---|---|---|---|
| Hospitalisation | |||||||||
| Patients with admissions (%) | 25.8 | 19.0 | 24.5 | 15.6 | 7.9 | 17.0 | 12.2 | 13.2 | 6.7 |
| Mean (SD) no of inpatient days a year | 27.9 (70.1) | 20.9 (66.8) | 20.6 (51.2) | 12.1 (46.1) | 5.7 (31.6) | 8.4 (33.1) | 10.5 (46.7) | 20.0 (71.8) | 5.0 (32.4) |
| Neurology | 8.1 | 7.0 | 10.4 | 3.0 | 1.2 | 2.8 | 1.0 | 0.6 | 0.7 |
| Rehabilitation | 11.1 | 8.3 | 7.6 | 5.3 | 0.8 | 3.2 | 6.1 | 3.9 | 1.1 |
| Nursing home | 6.6 | 4.6 | 1.4 | 2.3 | 3.4 | 1.5 | 2.4 | 14.2 | 2.0 |
| Consultations | |||||||||
| Mean (SD) no of medical and paramedical visits a year | 35.6 (58.0) | 37.5 (73.7) | 37.0 (50.8) | 28.8 (44.1) | 17.3 (28.1) | 46.5 (79.1) | 14.7 (28.7) | 21.2 (35.1) | 45.4 (36.6)) |
| Mean (SD) no of physiotherapy sessions a year | 11.6 (25.7) | 67.6 (89.9) | 41.4 (47.3) | 31.3 (48.1) | 25.5 (44.7) | 29.1 (52.5) | 13.9 (27.2) | 26.0 (38.9) | 8.2 (18.8) |
| Patients using DMD (%) | 39.8 | 49.7 | 50.3 | 42.6 | 35.5 | 52.4 | 42.6 | 37.9 | 20.6 |
| Investments, services | |||||||||
| Patients with investments (%) | 25.6 | 31.7 | 27.4 | 20.5 | 28.7 | 40.0 | 29.3 | 33.5 | 46.0 |
| Patients using services (%) | 24.5 | 37.3 | 25.0 | 30.6 | 40.6 | 28.2 | 40.4 | 39.5 | 19.8 |
| Informal care | |||||||||
| Patients using informal care (%) | 57.9 | 51.3 | 47.5 | 56.4 | 52.2 | 52.9 | 56.6 | 48.0 | 62.2 |
| Mean (SD) no of hours a year | 1024 (1956) | 664 (1521) | 497 (1175) | 1668 (2400) | 434 (1017) | 1341 (2453) | 498 (1064) | 601 (1514) | 1144 (2149) |
DMD, disease‐modifying drugs.
Costs
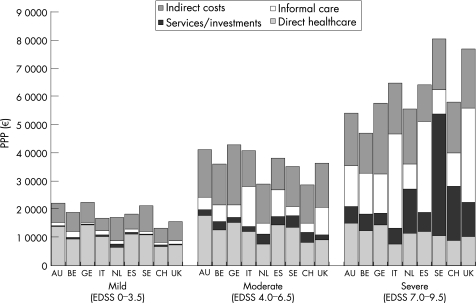
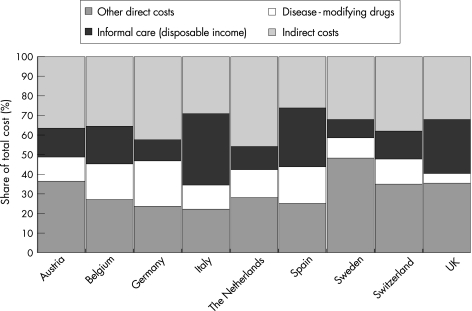
The total costs per patient are a function of both the quantity of a given resource used and its unit cost, as well as the patient sample included in the study; therefore, comparisons across countries should be made with great care. This is the case even in a study such as ours where both data collection and analytical methods have been standardised (except for the UK, where data handling and analysis were slightly different). Unless the results are linked to detailed prevalence data by severity, costs for the total population with multiple sclerosis and the mean cost per patient cannot be accurately estimated. Therefore, we present the overall results for patients with mild, moderate and severe disease (fig 3), proportions of costs represented by different resource types (fig 4) and more detailed consumption for given levels of disability (EDSS 2.0 and 6.5) as examples (table 3).
Figure 3 Patients are grouped into mild disease (Expanded Disability Status Scale (EDSS) 0–3.5), moderate disease (EDSS 4.0–6.5) and severe disease (EDSS 7.0–9.5), and total mean annual cost per patient (PPP) is calculated from the societal perspective. Local currencies have been transformed using the gross domestic product purchasing power parity index (OECD, 2004. Available at: www.oecd.org/std/ppp). Sweden and UK have the highest costs, particularly in advanced disease. In Sweden, this is clearly a consequence of the special service of personal assistants provided to people with disability, which, for example, in the severe patient group represents 43% of total costs, whereas in the UK, it is a consequence of a very high use of informal care associated with high revenues and a strong currency. AU, Austria; BE, Belgium; CH, Switzerland; ES, Spain; GE, Germany; IT, Italy; NL, The Netherlands; SE, Sweden; UK, United Kingdom.
Figure 4 Costs are presented as percentages of total cost per patient in each country, from the societal perspective (all costs, regardless of who pays). Other direct costs include all medical care (hospitalisation, consultations, tests, prescription and over‐the‐counter drugs, excluding disease‐modifying drugs), services (home care, home help, transportation) and investments (devices, appliances, changes to house and car). Informal care is calculated as loss of leisure time for the carer, using the disposable income (net after social contribution and taxes) as the cost of leisure time.
Table 3 Mean costs (€) per patient at Expanded Disability Status Scale (EDSS) 2.0 and EDSS 6.5 (2005).
| Austria | Belgium | Germany | Italy | The Netherlands | Spain | Sweden | Switzerland | UK | |
|---|---|---|---|---|---|---|---|---|---|
| Patients at EDSS 2.0 | |||||||||
| Utility | 0.719 | 0.649 | 0.721 | 0.677 | 0.694 | 0.717 | 0.696 | 0.767 | 0.725 |
| Total (SD) costs | 26 348 | 22 276 | 27 910 | 21 683 | 20 517 | 19 604 | 27 254 | 18 538 | 19 679 |
| (22 818) | (20 254) | (21 905) | (19 472) | (18 849) | (21 025) | (31 396) | (21 877) | (19 170) | |
| Direct costs | 18 132 | 13 582 | 18 305 | 15 067 | 9676 | 13 604 | 16 462 | 10 707 | 9537 |
| Inpatient care | 2979 | 1387 | 1807 | 1840 | 544 | 2240 | 4449 | 417 | 94 |
| Outpatient care | 4528 | 1902 | 2205 | 1215 | 1370 | 1131 | 2750 | 1419 | 3512 |
| Tests | 307 | 221 | 155 | 669 | 210 | 348 | 368 | 212 | 63 |
| Drugs | 8807 | 7426 | 12 881 | 7610 | 5027 | 7964 | 7459 | 6919 | 4324 |
| Services | 192 | 319 | 79 | 493 | 1176 | 155 | 139 | 518 | 102 |
| Investments | 133 | 466 | 171 | 169 | 259 | 231 | 211 | 120 | 160 |
| Informal care | 1185 | 1861 | 809 | 3071 | 1089 | 1534 | 1086 | 1102 | 1282 |
| Indirect costs | 8216 | 8694 | 9605 | 6616 | 10 842 | 6000 | 10 792 | 7831 | 10 142 |
| Patients at EDSS 6.5 | |||||||||
| Utility | 0.447 | 0.384 | 0.440 | 0.442 | 0.477 | 0.431 | 0.462 | 0.540 | 0.477 |
| Total (SD) costs | 54 821 | 43 790 | 55 344 | 53 717 | 44 196 | 44 104 | 52 457 | 49 274 | 53 724 |
| (31 791) | (25 295) | (20 761) | (32 016) | (26 744) | (30 198) | (47 567) | (37 477) | (41 532) | |
| Direct costs | 31 634 | 26 000 | 28 948 | 37 355 | 23 093 | 31 517 | 32 630 | 26 804 | 33 179 |
| Inpatient care | 7886 | 2784 | 4989 | 5316 | 2175 | 3284 | 6913 | 3652 | 1340 |
| Outpatient care | 6380 | 5194 | 4785 | 2196 | 2239 | 3564 | 3003 | 2461 | 5440 |
| Tests | 197 | 306 | 366 | 397 | 155 | 332 | 126 | 207 | 78 |
| Drugs | 7218 | 7724 | 7377 | 4089 | 4245 | 5505 | 5572 | 5904 | 2962 |
| Services | 742 | 2028 | 946 | 1702 | 4876 | 1714 | 8978 | 3479 | 2368 |
| Investments | 2189 | 2691 | 2769 | 1439 | 2564 | 1714 | 1592 | 6046 | 2609 |
| Informal care | 7022 | 8545 | 7715 | 22 215 | 6839 | 14 715 | 6445 | 7196 | 18 382 |
| Indirect costs | 23 187 | 17 790 | 26 396 | 16 362 | 21 103 | 12 588 | 19 827 | 22 471 | 20 545 |
Exchange rates: €1 = 9.07 SEK, 1.56 CHF, 0.688 GBP.
CHF, Swiss franc; GBP, British pound; SEK, Swedish kronor.
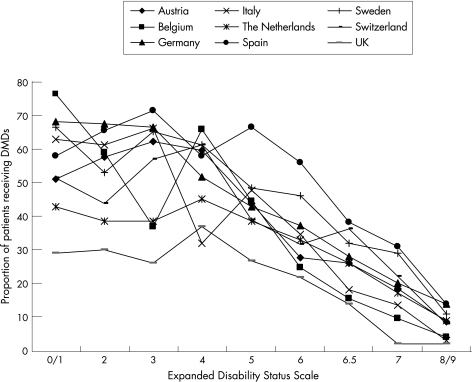
DMD usage varied between 21% (the UK) and 52% (Spain) among the countries, and was concentrated in early disease (fig 5). This had a considerable influence on the average cost per patient in the samples. It did not, however, influence other costs, as it was found that these were not different for patients with or without DMDs who were at the same level of EDSS and in the absence of a relapse. Thus, any effect on costs of these treatments will be through a reduced relapse rate and slower disease progression.
Figure 5 Treatment with disease‐modifying drugs (DMDs) as a proportion of patients treated at each level of Expanded Disability Status Scale (EDSS). DMDs include IFNβ‐1a (avonex), IFNβ‐1b (betaferon, rebif) and glatiramer acetate (copaxone). The Belgian sample at EDSS 3 appears as an outlier with low DMD treatment but potentially high relapse frequency, translating into low work capacity (fig 2), low utility (fig 6) and high costs.
The cost of a relapse of multiple sclerosis was similar across the countries, ranging between €2800 and €4000 with one exception (€5800 in Austria).
Utility and intangible costs
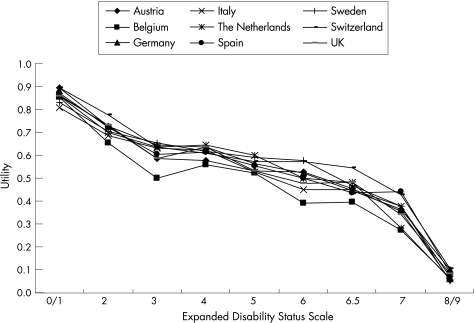
Unlike costs, utilities were almost identical across countries (fig 6), illustrating the consistency of disease definition across geographies and the strong correlation between disability and QoL. Similarly, the loss of utility during a relapse was comparable across countries, at around 0.1.
Figure 6 Utility scores are calculated with the original EuroQol—five domains (EQ‐5D) health status system in all countries24 to obtain comparable values. The EQ‐5D permits negative scores (ie, health states worse than death) and negative scores have been set to zero in our study.
The utility loss due to multiple sclerosis translated into a mean QALY loss of 0.27 (range 0.21–0.32) per patient and an intangible cost of €13 400 (range €10 300–15 400), assuming a willingness to pay for a QALY of €50 000. When three times GDP per capita is used as a threshold, the mean intangible cost is estimated at €19 800, with an expected larger variation (€12 100–33 000).
Discussion
To date, this is the largest study on burden of illness conducted on patients with multiple sclerosis. Resource consumption among patients is highly variable and the large samples included in the individual countries lead to less uncertainty in the results than in earlier analyses, with many issues that merit discussion.
The inclusion of several countries with historical differences in their approach to healthcare and disease presents a challenge. As data collection must be standardised to a large extent, there is a risk of losing information on some country‐specific particularities. The advantages of standardisation, however, prevail, and for the first time, it is possible to analyse differences between countries, whether due to the organisation of the healthcare systems, traditions, consumption, prices or the economic environment. All these factors strongly influence the outcomes, and even in a standardised study such as ours, it is not possible to compare the mean annual cost per patient without explaining the underlying differences.
We found differences among our national samples in patient demographics, consumption patterns, handling of early retirement and sick leave, and the price of individual resources, including the cost of employment. Analytical methods, usually one of the largest factors leading to differences in study results, have a limited role when comparing results among the countries in our study. They will, however, explain some of the differences compared with earlier studies.
Selecting a patient sample that is fully representative of the prevalence of a disease is difficult, because estimates of prevalence differ. The objective of this study was to estimate costs at each level of severity rather than to obtain a true population sample. Nevertheless, by adjusting to prevalence by severity, it is possible to estimate the total burden of the disease in each country.
Patients were recruited either through multiple sclerosis patient associations or through neurology clinics, and the two approaches may have led to slightly different samples. The average EDSS was 4.8 in the six countries where patients were recruited from patient associations compared with 3.9 in the three countries with samples from neurology clinics. This can be interpreted in two ways. Patient associations may include people with more advanced disease, as it may take some time for patients to join these associations and patients with more severe disease may be more likely to look for support. Neurology clinics, on the other hand, may see more patients who are in an earlier state of the disease process, particularly in multiple sclerosis where DMDs are indicated for relapsing disease and early treatment is advocated. Thus, such samples are likely to be biased towards an earlier state of disease. Indeed, only 6.8% of patients recruited in neurology clinics had an EDSS of 8 or higher, compared with 12.9% in the samples from patient associations. The truth may lie somewhere in between. None the less, as the main purpose of our study was to estimate costs and utility stratified by disease severity, the number of patients with multiple sclerosis at each EDSS level does not pose a problem for our analyses, because members of a patient association would not have more severe disease at a given EDSS level.
Another bias in the samples recruited by neurology clinics may arise from selecting patients on the dependent variable (consumption). Indeed, patients who are currently being followed up may be patients with a high consumption of healthcare resources. Our study, however, included all patients on file in the clinics rather than those with recent contacts. Consumptions should therefore be representative.
As expected, healthcare utilisation was quite variable across countries, reflecting differences in the organisation of healthcare systems, financial incentives, access and traditions. For instance, in countries where payment for hospitalisation is, or has until recently been, by a daily rate, both the number of admissions and the length of stay are substantially higher—for example, Austria, Belgium and Germany. Similarly, the number of medical visits is high in countries with more office‐based medical or paramedical practices that are easier to access, often combined with fee‐for‐service payment. Good examples of this are Belgium, Germany and Spain, which have a high frequency of both medical visits and physiotherapy sessions. Contrary to this, in countries where specialist consultations are mostly limited to hospitals, as in Sweden, or are capitated as in The Netherlands, the number of visits is lower. The proportion of patients who had to make investments or who required services such as home help or transportation was more similar across countries. It seems that these requirements are driven to a large extent by the disease and disease severity, with patients assuming all or part of the cost. The intensity of usage does differ, however, probably as a consequence of availability.
Informal care seems to be influenced by several factors. Although the proportion of patients who used informal care was strikingly similar across the samples, the number of hours was very different. Whereas patients in the three samples with earlier disease (Belgium, Germany and The Netherlands) received around 500 h of informal care per year, this amount was double or triple that seen in some of the countries with patients having more severe disease. This difference cannot be explained by a difference in disease severity alone. The amount of informal care is generally a function of the extent of services offered by the healthcare systems. A good example of this is Sweden, where patients with severe disability have access to personal assistants who are funded by the system. As a consequence, the use of services is highest in Sweden, whereas the use of informal care is among the lowest, despite the fact that the sample has the highest EDSS. Contrary to this, countries such as Italy or the UK seem to offer limited services, leading to a greater use of informal care. Family structure also seems to have an influence on usage. In countries where fewer women are employed outside the home, informal care is more readily available and usage is therefore higher. Also, in these countries, fewer patients live alone (around 10–12% in Italy and Spain compared with >25% in some of the other countries). Similar results were found in some of the earlier studies.14,15,16,19 Lastly, people from different cultures may understand the concept of informal care in different ways.
Finally, the cost per patient is driven by quantities of resources used and their prices, and these prices differ across countries and settings. In countries with a national health system, such as Sweden and the UK, unit costs are derived from overall usage and are likely to represent opportunity costs, whereas in fee‐for‐service systems they represent tariffs that may include other incentives. In general, unit costs are considerably higher—for example, the cost of a medical consultation with a neurologist in Sweden or the UK costs more than €200 compared with €19.18 in Germany. Quantities, prices and availability also drive the share of costs represented by different types of resources (fig 4).
Productivity losses still represent the single highest contributor to societal costs, but the proportion is lower than that found in studies in the early 1990s, when direct costs were very low. Despite this, costs borne by payers such as the healthcare sector or social services represent only around half of the total cost per patient in most countries.
All information in our study was collected from patients, with no opportunity to clarify or verify responses because the answers were anonymous. We have, however, shown earlier that reporting by patients is highly accurate despite potential cognitive difficulties in this patient group and general recall bias.19 For instance, in a study in Germany, the difference between the mean number of hospitalisation days reported by 200 patients with multiple sclerosis and the mean abstracted from their medical records was half a day.19
Patients were also asked to assess their EDSS on the basis of descriptions from the original instrument focusing primarily on ambulation.21 This may seem to be a limitation in the validity, but a pre‐test of the questionnaire on a small sample had shown a good correlation with EDSS, and in particular the Patient Determined Disease Steps has a very high correlation.22 Patients seemed to have difficulties in distinguishing between relapsing and progressive disease, however, and we did not include this distinction in the analysis. Earlier studies have shown that, when controlling for relapses, costs were not different for patients with different courses of multiple sclerosis at the same level of EDSS.5
A striking finding in our study is that utility scores by EDSS are virtually the same across the countries. This confirms the overwhelming effect of multiple sclerosis on QoL, but is obviously also the consequence of the fact that the same health state tariff for the utility instrument (EQ‐5D) was used.24 Over 1 year, patients lose on average a third of a QALY compared with the general population, leading to an average cost increase of €15 000 or 30% owing to intangible costs.
Conclusion
Costs for patients with multiple sclerosis in Europe increase more than threefold to fourfold in patients with severe disease (EDSS >7.0) compared with patients with an earlier disease state (EDSS <4.0); the effect of advancing disease is detrimental on QoL. Drugs that slow disease progression early on, thus avoiding or delaying the severe disease states in which patients are unable to work and become dependent on help from their family, will provide large benefits to society.
Acknowledgements
We thank MedSciNet for constructing and managing the internet database; Jeroen Weites and Biogen Idec for project assistance; and Ulrika Lilja and Oskar Ström from Stockholm Health Economics for help with analyses.
Abbreviations
DMD - disease‐modifying drugs
EDSS - Expanded Disability Status Scale
EQ‐5D - EuroQol
GDP - gross domestic product
QALY - quality‐adjusted life year
QoL - quality of life
Footnotes
Funding: This project was supported by an unrestricted grant from BiogenIdec, Cambridge, Massachusetts, USA, and Elan Corporation, San Diego, California, USA.
Competing interests: None declared.
The following investigators or societies participated in this study. Austria—Austrian Multiple Sclerosis Society; Belgium—CU St Luc, Woluwe‐Saint Lambert: Prof Christian Sindic, Dr Sophie Goffette; Centre Neurologique & Réadaptation Fonctionnelle, Fraiture: Dr D Guillaume, Dr R Reznik; MS & Revalidatiecentrum, Overpelt: Dr Luc Vande Gaer, Dr Eric de Smet; Elisabeth Ziekenhuis, Sijsele: Dr Danny Decoo; Germany—Hamburg: Dr Wolfgang‐Gerhard Elias; Quellenhof Neurologisches Rehabilitationszentrum, Bad Wildbad: Prof Peter Flachenecker; Kaltenkirchen: Dr Matthias Freidel; Marianne‐Strauss‐Klinik, Berg: Prof Nicolaus König; Universitätsklinikum Essen, Essen: Prof Volker Limmroth; Barsinghausen: Dr Elmar Straube; Italy—University of Genvoa, Genova: Dr Antonio Uccelli; Associazione Italiana Sclerosi Multipla (AISM): Prof Mario A Battaglia; ADIS International, Milano: Dr Carlo Lucioni; The Netherlands—VU Medical Center, Amsterdam: Prof Chris Polman, Dr Bernard Uitdehaag; MS Center Nijmegen: Dr Peter JH Jongen; Maasland Ziekenhuis, Sittard: Dr HW Anten; Spain—Hospital Virgen de la Macarena, Sevilla: Dr Guillermo Izquierdo; Biogen Idec Iberia, S.L., Madrid: Dr Olga Sánchez‐Soliño, Dr Juan Pérez Miranda; PORIB, Madrid: Dr Miguel Angel Casado; Asociación Española De Esclerosis Múltiple; Sweden—Karolinska Hospital Stockholm: Prof Sten Fredrikson; Neurologisk Handikappades Riksförbund‐Annica Bernehjält; Switzerland—Schweizerische MS Gesellschaft, Zurich: Dr Judy Lutz; UK—Heron Evidence Development Ltd, Letchworth: Dr David Tyas, Mr John Kerrigan; Multiple Sclerosis Trust.
References
- 1.Parkin D, Jacoby A, McNamee P. Treatment of multiple sclerosis with interferon beta‐1b: an appraisal of cost‐effectiveness and quality of life. J Neurol Neurosurg Psychiatry 200068144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes R B, Lees A, Waugh N.et al Population based cost utility study of interferon beta‐1b in secondary progressive multiple sclerosis. BMJ 19993191529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobelt G, Jönsson L, Henriksson F.et al Cost‐utility of interferon beta‐1b in secondary progressive multiple sclerosis. Int J Technol Assess Health Care 200016768–780. [DOI] [PubMed] [Google Scholar]
- 4.Kobelt G, Jönsson L, Miltenburger C. Cost‐utility analysis of interferon‐beta‐1b in secondary progressive multiple sclerosis, using natural history disease data. Int J Technol Assess Health Care 200218127–138. [PubMed] [Google Scholar]
- 5.Kobelt G, Jönsson L, Fredrikson S. A new disease model to estimate the cost of disease progression for different types of MS and different subgroups of patients. Eur J Health Econ 2003450–59. [DOI] [PubMed] [Google Scholar]
- 6.Nuijten M, Hutton J. Cost‐effectiveness of beta interferon in multiple sclerosis: a Markov process analysis. Value Health 2002544–54. [DOI] [PubMed] [Google Scholar]
- 7.Chilcott J, McCabe C, Tappenden P.et al Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. BMJ 2003326522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LePen C, Coyle P, Vollmer T.et al Long‐term cost effectiveness of interferon‐beta‐1a in the treatment of relapsing remitting multiple sclerosis. Clin Drug Invest 200323571–581. [DOI] [PubMed] [Google Scholar]
- 9.Prosser L, Kuntz K, Bar‐Or A.et al Cost effectiveness of interferon beta‐1a, interferon beta‐1b and glatiramer acetate in newly diagnosed non primary progressive multiple sclerosis. Value Health 20047554–568. [DOI] [PubMed] [Google Scholar]
- 10.Blumhardt L, Wood C. The economics of multiple sclerosis: a cost of illness study. Br J Med Econ 19961099–118. [Google Scholar]
- 11.Holmes J, Madgwick T, Bates D. The cost of multiple sclerosis. Br J Med Econ 19958181–193. [Google Scholar]
- 12.Henriksson F, Jönsson B. The economic cost of multiple sclerosis in Sweden in 1994. Pharmacoeconomics 199813597–606. [DOI] [PubMed] [Google Scholar]
- 13.Carton H, Loos R, Pacolet J.et al Utilisation and cost of professional care and assistance according to disability of patients with multiple sclerosis in Flanders (Belgium). J Neurol Neurosurg Psychiatry 199864444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amato M P, Battaglia M A, Caputo D.et al The costs of multiple sclerosis: a cross‐sectional, multicenter cost‐of‐illness study in Italy. J Neurol 2002249152–163. [DOI] [PubMed] [Google Scholar]
- 15.Henriksson F, Fredrikson S, Masterman T.et al Costs, quality of life and disease severity in multiple sclerosis. A cross‐sectional study in Sweden. Eur J Neurol 2001827–35. [DOI] [PubMed] [Google Scholar]
- 16.Kobelt G, Lindgren P, Parkin D.et alCosts and quality of life in multiple sclerosis. A cross‐sectional observational study in the United Kingdom. Stockholm: Stockholm School of Economics, 2000
- 17.Russo P, Capone A, Paolillo A.et al Cost analysis of relapsing‐remitting multiple sclerosis in Italy after the introduction of new disease‐modifying agents. Clin Drug Invest 200424409–420. [DOI] [PubMed] [Google Scholar]
- 18.Kazas E, Grisouard R, Zanni J.et al Multiple sclerosis treatment with interferon beta: prevalence and cost of the public health fund in 2000. Rev Méd Assurance Maladie 200334147–156. [Google Scholar]
- 19.Kobelt G, Lindgren P, Smala A.et al Costs and quality of life in multiple sclerosis. A cross‐sectional observational study in Germany. Eur J Health Econ 2001260–68. [Google Scholar]
- 20.Kobelt G, Berg J, Atherley D.et alCost and quality of life in multiple sclerosis. A cross‐sectional study in the USA. EFI Research Report Number 594. Stockholm: Stockholm School of Economics, 2005
- 21.Kurtzke J. Rating neurological impairment in multiple sclerosis and Expanded Disability Status Scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 22.Hohol M, Orav E, Weiner H. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 199545251–255. [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Group EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 199016199–208. [DOI] [PubMed] [Google Scholar]
- 24.Dolan P, Gudex C, Kind P.et alA social tariff for EuroQol: results from a UK general population survey. Discussion Paper Number 138. York: Centre for Health Economics, University of York, 1995
- 25.Torrance G. Measurement of health state utilities for economic appraisal. A review. J Health Econ 198651–30. [DOI] [PubMed] [Google Scholar]
- 26.Koopmansschap M, Rutten F, van Ineveld B.et al The friction cost method for measuring indirect costs of disease. J Health Econ 199514171–189. [DOI] [PubMed] [Google Scholar]
- 27.Eichler H, Kong S, Gerth W C.et al Use of cost‐effectiveness analysis in health care resource allocation decision‐making: how are cost‐effectiveness thresholds expected to emerge? Value Health 20047518–528. [DOI] [PubMed] [Google Scholar]