Abstract
The intrathecal synthesis of IgM, determined at clinical onset in patients with multiple sclerosis, was found to correlate with the degree of disability (as evaluated by means of the Expanded Disability Status Scale) reached 15 years later (p<0.001). Moreover, a significant inverse correlation was observed between the value of the IgM index and time to the first relapse (p<0.001) and the initiation of the progressive phase of the disease (p = 0.01). The prognostic value of IgM in the CSF is confirmed in previous reports as well as by our study. If these findings are confirmed in patients with multiple sclerosis in a larger series, a helpful biological marker for selecting patients for immunomodulatory treatments will be available to neurologists.
Intrathecally synthesised IgM can be seen in the CSF of infectious and inflammatory diseases of the CNS.1,2,3 Increased IgM index and locally produced IgM oligoclonal bands have been observed in the CSF of patients with multiple sclerosis as well.4,5,6 Data from Villar et al7,8,9,10 suggest that IgM may have a role in the evolution of the disease, at least in a subgroup of patients. Moreover, IgM in the CSF of patients with multiple sclerosis was found to correlate with the intrathecal synthesis of C311 and with the local concentration of myelin basic protein.6
While looking for biological markers for multiple sclerosis, in the early 1990s we focused our attention on IgM in the CSF of patients with multiple sclerosis at the onset of disease. IgM concentration in paired CSF and serum specimens was measured by ELISA.12 Owing to the high frequency with which unspecific or false‐positive banding was detected by means of the amplification system used (avidin–biotin–peroxidase staining after protein transfer to nitrocellule membrane and IgM (Fc) immunofixation), the demonstration of IgM oligoclonal bands by isoelectric focusing was soon abandoned. An increased IgM index (ie, CSF IgM/serum IgM:CSF albumin/serum albumin) was, however, found in 65–70% of the CSF collected for diagnostic purposes after the first episode of neurological dysfunction suggestive of multiple sclerosis. No correlation between IgM in the CSF and patient's age and sex, age at the onset of disease, type of clinical presentation, MRI picture and other CSF parameters was observed. Therefore, the IgM index was not incorporated in routine CSF analysis.
Considering the possible prognostic value of IgM9,10 and that a role for B cells in the pathogenesis of multiple sclerosis is strongly supported by immunological and pathological evidence,13,14,15 we reconsidered our IgM data and verified a possible relationship between IgM index values at the onset of disease and the clinical evolution of the disease, by evaluating the actual clinical status of the patients.
Patients, methods and results
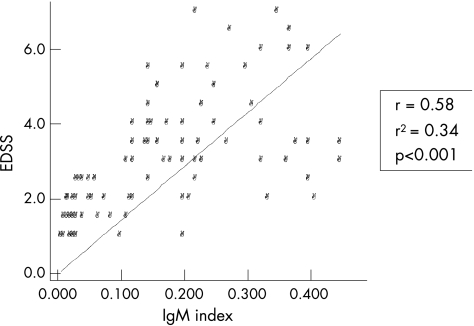
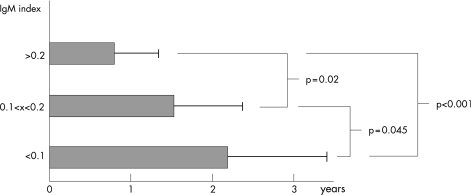
In all, 80 patients with multiple sclerosis (54 (67.5%) women; 26 (32.5%) men; female:male 2.07), randomly selected among patients in whom lumbar puncture was done within 1 month from clinical onset of the disease, were considered. All samples were collected during the period 1989–93 and stored at −80°C in aliquots until tests were carried out in 1994. As mentioned, IgM was detected in paired CSF and serum specimens by ELISA, following a method previously published,12 with only minor modifications. Mean disease duration from clinical onset (and CSF sampling) to the last Expanding Disability Status Scale (EDSS) examination was 14.4 (SD 2.1) years. IgM index was normal (<0.1) in 25 of 80 (31.35%) patients and increased in 55 of 80 (68.75%) patients. A significant correlation was observed between IgM index and EDSS (Spearman, r = 0.58, r2 = 0.34, p<0.001; fig 1). All patients with IgM index <0.1 had an EDSS score ⩽3 and a relapsing–remitting multiple sclerosis course (mean disease duration 13.8 (SD 1.4) years). Of these, only 7 of the 25 patients had been treated with immunomodulatory agents, whereas none received immunosuppressive drugs. Therefore, the so far less aggressive clinical course of the disease in these patient groups was not because of treatments based on disease‐modifying agents. Although some patients (12/80) with increased IgM showed EDSS values <3 (8 received immunomodulatory agents), all patients with EDSS ⩾3 had an increased IgM index, and all the patients (10/80) with EDSS ⩾5.5 (at present, all of them are in the secondary progressive phase of the disease) had particularly high IgM index values (⩾0.2, in our setting). Moreover, when time to the first relapse (ie, conversion to clinically definite multiple sclerosis) was calculated, patients with an IgM index >0.2 had a noticeably shorter time to the first relapse (mean 0.75 (SD 0.5) years) compared with patients with normal IgM index values (mean 2.2 (SD 1.15) years, p = 0.001; fig 2). The time to the initiation of secondary progressive phase of the disease also correlates with IgM index value (p = 0.01). Indeed, 65% of patients with IgM values >0.2 converted into secondary progressive multiple sclerosis during the follow‐up period.
Figure 1 A significant correlation between intrathecal IgM synthesis (IgM index) at the onset of disease and disease progression (ie, Expanding Disability Status Scale (EDSS) after a mean disease duration of 14.4 (SD 2.1) years) was found in 80 patients with multiple sclerosis.
Figure 2 The time to the first relapse (TFR) in patients with relapsing–remitting multiple sclerosis was found to be inversely correlated with IgM index values. A high significance was observed between patients with normal IgM index (TFR = 2.2 (SD 1.15) years) and those with IgM index >0.2 (TFR = 0.75 (SD 0.5) years, p<0.001).
Discussion
Our findings suggest that the lower the IgM index is at the clinical onset of multiple sclerosis, the slower the progression of the disease is in the following 15 years, strongly confirming previously reported observations regarding the predictive value of intrathecally synthesised IgM in patients with multiple sclerosis.7,8 Indeed, Villar et al8 reported that 90% of patients with multiple sclerosis with IgM oligoclonal bands in the CSF at clinical onset had a relapse in the following 8 months, whereas IgM‐negative patients converted to clinically definite multiple sclerosis in a much longer period (51% converted in 36 months). These findings are confirmed in our study, in which patients with the highest IgM index values had a mean time to the first relapse of 9 months, whereas in IgM‐negative patients time to the first relapse was 2.2 (SD 1.15) years. The same authors7 found that none of the patients without intrathecal synthesis of IgM converted into secondary progressive multiple sclerosis and showed an EDSS score <3 during a follow‐up period ranging from 5 to 16 years, whereas all patients with a “non‐benign” multiple sclerosis had increased IgM in the CSF at clinical onset. These findings are also confirmed by our study. In the work of Villar et al, 70.8% of patients with intrathecal IgM synthesis converted to secondary progressive multiple sclerosis during the follow‐up, whereas none of the patients without intrathecal IgM synthesis did. At the end of the study, 63.6% of IgM‐positive patients had reached EDSS = 6, whereas none of the patients lacking IgM in the CSF reached EDSS values >3. When patients with a “benign” multiple sclerosis course were analysed, 82% lacked IgM, whereas all patients with a non‐benign multiple sclerosis had an intrathecal synthesis of IgM. The less aggressive clinical course of our patients can be partially explained by the beneficial effect of immunomodulatory agents. Indeed, most (66.6%) patients with IgM index >0.2 and EDSS <3.0 were treated with interferon β.
Although no correlation was found between IgM and white matter lesions on MRI (as disclosed by means of T2, DP, T1 and gadolinium‐enhanced standard sequences) at onset, a study aimed at the identification of differences in CNS pathology (including cortical thickness and lesions) between patients with IgM‐positive and IgM‐negative values is recommended. From the immunopathological point of view, it may be possible that IgM in the CSF are naturally occurring antibodies that recognise myelin antigens,10 or the expression of a persistent primary antibody response to cross‐reacting antigens mediated by CD19CD5 cells.16,17 We must point out that IgM are strong complement activators, and that the deposition of antibodies and complement factors in inflammatory white matter lesions correlates with a more severe demyelination and axonal loss.14,18
As no prognostic biological markers are currently available for multiple sclerosis, we recommend further studies on the intrathecal IgM synthesis. Indeed, whatever the pathological significance of IgM in the CSF, if their prognostic value is confirmed, the neurologist will have a helpful tool for selecting patients for early immunomodulatory agents‐based treatments.
Abbreviations
EDSS - Expanding Disability Status Scale
Footnotes
Competing interests: None declared.
References
- 1.Forsberg P, Fryden A, Link H.et al Viral IgM and IgG antibody synthesis within the central nervous system in mumps meningitis. Acta Neurol Scand 198673372–380. [DOI] [PubMed] [Google Scholar]
- 2.Chiodi F, Sundqvist V A, Norrby E.et al Measles IgM antibodies in cerebrospinal fluid and serum in subacute sclerosing panencephalitis. J Med Virol 198618149–158. [DOI] [PubMed] [Google Scholar]
- 3.Chiodi F, Sunqvist V A, Link H.et al Viral IgM antibodies in serum and cerebrospinal fluid in patients with multiple sclerosis and controls. Acta Neurol Scand 198775201–208. [DOI] [PubMed] [Google Scholar]
- 4.Walsh M J, Torutellotte W W. Temporal invariance and clonal uniformity of brain and cerebrospinal fluid IgG, IgA and IgM in MS. J Exp Med 198616341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharief M K, Thompson E J. Intrathecal immunoglobulin M synthesis in multiple sclerosis. Brain 1991114181–195. [PubMed] [Google Scholar]
- 6.Frequin S T F M, Barkhof F, Lamers K J B.et al CSF myelin basic protein, IgG and IgM levels in 101 MS patients before and after treatment with high‐dose intravenous methylprednisolone. Acta Neurol Scand 199286291–297. [DOI] [PubMed] [Google Scholar]
- 7.Villar L M, Masjuan J, Gonsalez‐Porqué P.et al Intrathecal IgM synthesis in neurological diseases. Relationship with disability in MS. Neurology 200258824–826. [DOI] [PubMed] [Google Scholar]
- 8.Villar L M, Masjuan J, Gonsalez‐Porqué P.et al Intrathecal IgM synthesis predicts the onset of new relapses and a worse disease course in MS. Neurology 200259555–559. [DOI] [PubMed] [Google Scholar]
- 9.Villar L M, Masjiuan J, Gonzalez‐Porque P.et al Intrathecal IgM synthesis is a prognostic factor in MS. Ann Neurol 200353222–226. [DOI] [PubMed] [Google Scholar]
- 10.Villar L M, Sabada M C, Roldan E.et al Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest 2005115187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellebjerg F, Christiansen M, Garret P. MBP, anti‐MBP and anti‐PLP antibodies, and intrathecal complement activation in MS. Mult Scler 19984127–131. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser R, Lücking C H. Intrathecal synthesis of IgM and IgA in neurological diseases: comparison of two formulae with isoelectric focusing. Clin Chim Acta 199321639–51. [DOI] [PubMed] [Google Scholar]
- 13.Corcione A, Casazza S, Ferretti E.et al Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci USA 200410111064–11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lassmann H, Bruck W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 20017115–121. [DOI] [PubMed] [Google Scholar]
- 15.Kornek B, Lassmann H. Neuropathology of multiple sclerosis – new concepts. Brain Res Bull 200361321–326. [DOI] [PubMed] [Google Scholar]
- 16.Berland R, Wortis H H. Origin and functions of B‐1 cells with notes on the role of CD5. Ann Rev Immunol 200220253–300. [DOI] [PubMed] [Google Scholar]
- 17.Mix E, Olsson T, Correale J.et al B cells expressing CD5 are increased in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol 19997921–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead R J, Singhrao S K, Neal J W.et al The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol 2002168458–465. [DOI] [PubMed] [Google Scholar]