Abstract
Background
Huntington's disease is a progressive neurodegenerative disorder that results in deterioration and atrophy of various brain regions.
Aim
To assess the functional connectivity between prefrontal brain regions in patients with Huntington's disease, compared with normal controls, using functional magnetic resonance imaging.
Patients and methods
20 patients with Huntington's disease and 17 matched controls performed a Simon task that is known to activate lateral prefrontal and anterior cingulate cortical regions. The functional connectivity was hypothesised to be impaired in patients with Huntington's disease between prefrontal regions of interest, selected from both hemispheres, in the anterior cingulate and dorsal lateral prefrontal cortex.
Results
Controls showed a dynamic increase in interhemispheric functional connectivity during task performance, compared with the baseline state; patients with Huntington's disease, however, showed no such increase in prefrontal connectivity. Overall, patients with Huntington's disease showed significantly impaired functional connectivity between anterior cingulate and lateral prefrontal regions in both hemispheres compared with controls. Furthermore, poor task performance was predicted by reduced connectivity in patients with Huntington's disease between the left anterior cingulate and prefrontal regions.
Conclusions
This finding represents a loss of synchrony in activity between prefrontal regions in patients with Huntington's disease when engaged in the task, which predicted poor task performance. Results show that functional interactions between critical prefrontal regions, necessary for cognitive performance, are compromised in Huntington's disease. It is speculated whether significantly greater levels of activation in patients with Huntington's disease (compared with controls) observed in several brain regions partially compensate for the otherwise compromised interactions between cortical regions.
Huntington's disease is a progressive autosomal dominant neurodegenerative disorder that leads to dementia and death about 15–20 years from diagnosis.1,2 Patients with Huntington's disease are impaired in cognitive, emotional and motor functioning, and develop chorea.3 Clinical onset is typically around 35–44 years of age. Although various subcortical and cortical regions have been identified in the neuropathology of Huntington's disease, we still do not understand the functional connectivity between regions and how this may change with deterioration over time. Functional magnetic resonance imaging (fMRI) provides a method by which functional connectivity, or interactions between disparate brain regions, can be examined.
Huntington's disease results in neurodegeneration and atrophy of several brain regions including the striatum,4 anterior cingulate, lateral prefrontal and parietal regions.5 These cortical regions are all connected with the striatum (caudate and putamen) via parallel partially overlapping corticostriatal circuits.6 In Huntington's disease, it is widely believed that the frontal associative and motor circuits are disrupted, with loss of neurones in the striatum contributing to the cognitive and motor dysfunctions.7,8 Executive functions are also compromised, most probably owing to loss of connections between the caudate and frontal lobes9 as well as the limbic circuit involving the anterior cingulate.10
Although previous studies have shown specific impairment of parts of these circuits in Huntington's disease, changes in the functional connectivity or interactions between prefrontal circuits remain unclear. Functional brain imaging methods, such as fMRI, have disclosed specific changes in prefrontal cortical function during cognitive task performance in symptomatic and presymptomatic patients with Huntington's disease.10,11 In particular, Paulsen et al10 reported relative hyperactivation in prefrontal regions in presymptomatic patients with Huntington's disease compared with controls, which they suggest may compensate for striatal degeneration. However, no fMRI study has examined the functional interactions between prefrontal cortical areas in Huntington's disease.
Interactions between brain regions can be measured as functional connectivity,12 defined as correlations of fMRI blood‐oxygen level‐dependent (BOLD) signal responses between brain regions.13 Functional connectivity identifies regions that are synchronously active independent of task manipulations. In contrast with fMRI activation studies, which focus only on activation within individual brain regions, functional connectivity can determine inter‐regional relationships.14 Measuring connectivity in this way is useful when assessing brain regional pathology in Huntington's disease, as the connections between disparate regions may be impaired owing to loss of function from neuronal cell death.15 Indeed, previous studies of functional connectivity in progressive and neurodegenerative conditions, such as Alzheimer's and Parkinson's diseases, typically show reduced connectivity in patients, providing additional insight into brain regional pathology.16,17
This study aimed to examine functional connectivity within the prefrontal cortex in symptomatic patients with Huntington's disease compared with controls. We used fMRI to examine functional connectivity during the performance of a Simon task, which we have previously shown to discriminate patients with Huntington's disease and controls behaviourally.18
The Simon task is a cognitive interference task involving spatially congruent compared with incongruent stimulus–response mappings and has been shown to involve activation of frontostriatal brain regions, particularly lateral prefrontal cortex and anterior cingulate areas, as well as the inferior parietal cortex.19 In an fMRI study, we report that the Simon task shows differential increased patterns of BOLD activation in patients with Huntington's disease compared with controls (Georgiou‐Karistianis et al, submitted for publication) in the anterior cingulate and lateral prefrontal regions; these regions will therefore form the regions of interest (ROI) for this study. Indeed, other studies have also reported increased patterns of activation during learning tasks in presymptomatic patients with Huntington's disease.20 In this study, we examined functional connectivity between anterior cingulate and lateral prefrontal cortical regions, and hypothesised that patients with Huntington's disease would show impaired functional connectivity between these regions owing to the known prefrontal pathology associated with this disease.
Methods
Participants
In all, 20 patients with Huntington's disease (mean (standard deviation (SD)) age 47.9 (7.7) years, range 36–63 years) and 17 controls (mean (SD) age 49.7 (7.3) years, range 37–62 years) were recruited. The patients' duration of illness ranged from 1 to 11 years with CAG repeat length ranging from 36 to 51 (mean (SD) 43.4 (3.4)), thus representing a heterogeneous group. The duration of illness for each patient with Huntington's disease was determined as the period between initial symptoms as determined retrospectively by the treating clinician to the time when they were scanned. For patients with Huntington's disease, a measure that is used to investigate the influence of varying CAG repeats on brain function, corrected for age, was calculated using the equation21
CAG index=age×(CAG−35.5)
This measure reflects the severity of Huntington's disease as it has been found to correlate with bicaudate diameter ratio and scores of symptom severity.22 In this study, the CAG index ranged from 29.5 to 666.5 (mean (SD) 363 (136)). The controls had no history of a neurological or psychiatric disorder. All patients had normal or corrected to normal vision, and all but one were right handed. A neurologist (AC) or neuropsychiatrist (PC) administered the motor subscale of the Unified Huntington's Disease Rating Scale (UHDRS)23 to assess current motor symptom severity. The patients' UHDRS motor scores ranged from 8 to 55 (mean (SD) 23.1 (13.7)). Three patients were not receiving any drugs. The other 17 patients were using one or more of antidepressants, antipsychotics or mood stabilisers, with one patient on a cholesterol‐lowering agent. The Mini‐Mental State Examination24 was used to assess cognitive decline, the National Adult Reading Test25 was used to assess estimated premorbid IQ and the Beck Depression Inventory26 was administered to assess depressive symptoms (often observed in Huntington's disease). One‐way analysis of variance showed no significant group differences in estimated IQ (f1,31 = 2.85, p = 0.10). However, the Huntington's disease group had significantly higher Beck Depression Inventory scores (f1,34 = 9.99, p<0.005) and significantly lower Mini‐Mental State Examination scores (f1,33 = 10.26, p<0.005; table 1), although scores were within the normal range. This study was approved by the Human Research Ethics Committees of Monash University and the Howard Florey Institute, Victoria, Australia, and written consent was obtained from all participants.
Table 1 Demographic and clinical data for controls and patients with Huntington's disease.
| Controls | Patients with HD | |
|---|---|---|
| Male/female | 15/2 | 19/1 |
| Age (years) | 49.7 (7.3) | 47.9 (7.7) |
| UHDRS | — | 23.1 (13.7) |
| Duration of illness (years) | — | 5.4 (3.6) |
| CAG repeats | — | 43.4 (3.4) |
| CAG index | — | 363 (136) |
| MMSE | 29.2 (1.3) | 27.1 (2.4) |
| IQ | 119 (6.1) | 115.5 (6.1) |
| BDI | 1.5 (1.7) | 6.9 (6.9) |
–, test not administered; BDI, Beck Depression Inventory score (maximum score 63, normal range 0–9); CAG, cytosine–adenine–guanine; HD, Huntington's disease; IQ, estimated full‐scale IQ calculated from National Adult Reading Test error score; MMSE, Mini‐Mental State Examination score (maximum score 30); UHDRS, motor subscale score from the Unified Huntington's Disease Rating Scale.
Values are mean (SD).
Experimental paradigm
During MRI scanning, participants viewed stimuli projected on to a screen at the foot of the MRI scanner bed via a mirror mounted inside the scanner head coil. Participants held a button box in each hand and were required to respond to stimuli by pressing left or right buttons. The Simon task consisted of single arrows presented either on the left or right of the display and pointing either to the left or the right (fig 1). The participant's task was simply to press the button on the side to which the arrow was pointing. Congruent trials involved left‐positioned arrows pointing left and right‐positioned arrows pointing right. Incongruent trials involved arrows positioned on the right pointing to the left (requiring a left‐hand response) and arrows positioned on the left pointing to the right (requiring a right‐hand response), so the spatial location of the stimulus and the side of response were incongruent. Each arrow was presented for 2500 ms, followed by a 500 ms duration blank screen. Participants' response times were recorded from the onset of stimulus presentation to the button‐press response. Errors consisting of the wrong side of response were also recorded. Participants were instructed to respond as quickly and as accurately as possible. The participants were given a break in between sessions to minimise effects of fatigue or boredom.
Figure 1 The Simon task stimuli. Participants respond by pressing a left or right button according to the direction of the arrowhead. The spatial location of the arrow stimulus and the side of response are either congruent (top) or incongruent (middle). A baseline condition (bottom) involves no congruency effect and requires no response.
Throughout MRI scanning, performance of the Simon task was alternated with a baseline condition in a blocked‐design fMRI paradigm. The baseline condition consisted of two horizontal lines presented concurrently to the left and right of fixation. Participants were required to maintain fixation but not make any button‐press response during baseline trials. Each participant completed four MRI measurement series of approximately 8 min each. Each session started with a baseline block (four trials: 12 s duration) followed by a task block (six trials: 18 s duration), and continued with 16 alternating baseline/task blocks in total (96 congruent and 96 incongruent trials per session). Over all four sessions, each participant performed a total of 192 congruent and 192 incongruent trials, randomly mixed within blocks.
Data acquisition and analysis
A 3.0 T GE Signa whole‐body scanner (General Electrics, Milwaukee, WI, USA) was used to record gradient‐echo echo‐planar images (TR = 3000 ms, TE = 40 ms, FA = 60°, FOV = 24 cm, matrix size 128×128). To cover the entire brain, 25 transaxial slices with a slice thickness of 5.0 mm and a gap of 0.5 mm were acquired. T2‐weighted and T1‐weighted anatomical images were also acquired. All image processing and analysis was performed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK). Firstly, all images within each measurement series were realigned to the first image to correct for participant's head motion during acquisition. The mean functional image for each participant was then coregistered to their own T2‐weighted anatomical image, and the T2‐weighted image was spatially normalised to the SPM2 template image (reference brain of the Montreal Neurological Institute, in approximate Talairach coordinate space). A combined normalisation matrix was then applied to all functional images for each participant, resulting in resliced images with voxel size 2×2×2 mm3. Images were then spatially smoothed using an 8×8×8 mm3 isotropic gaussian kernel.
Head motion for 35 participants was <1.5 mm translation and <2° rotation. Two participants showed between 1.5 and 2 mm translation. Individually examining head motion over time in each participant showed no task‐correlated motion.
For statistical analysis of task‐related activation patterns, the Simon task condition (congruent and incongruent combined) alternating with baseline was modelled, together with head motion correction parameters, in a general linear model analysis. Independent t tests were used to identify regions showing significant differences in activation in patients with Huntington's disease compared with controls. Activation results associated with this task will be published separately10 (Georgiou‐Karistianis et al, submitted for publication). The purpose of the statistical analysis described here was to functionally localise ROI in the prefrontal cortex, as regions showing differences in task‐related activation, for examination of prefrontal functional connectivity.
ROI selection
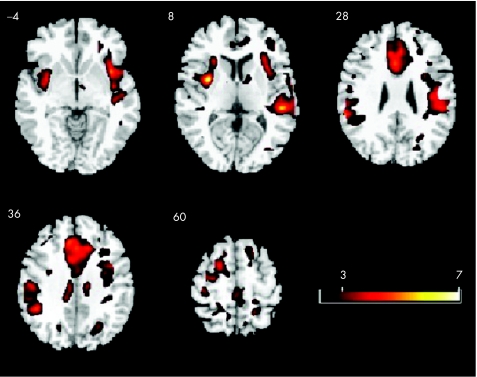
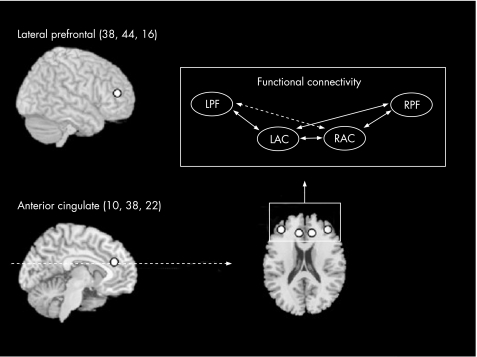
Statistical activation maps showed that, overall, patients with Huntington's disease recruited a larger number of cortical areas than controls to perform the Simon task (fig 2). Hence, the patients with Huntington's disease showed significantly greater activation than controls bilaterally in the caudal anterior cingulate gyrus, right dorsal premotor cortex, right inferior frontal gyrus, left middle frontal gyrus, and left superior parietal lobule. As our aim was to examine prefrontal connectivity, we selected peak voxels from these activation results within the anterior cingulate (left/right: coordinates (mm) ±10, 38, 22) and lateral prefrontal regions on the border of inferior and middle frontal gyri (left/right: coordinates (mm) ±38, 44, 16) to model prefrontal functional connectivity (fig 3). ROIs were defined as 6 mm diameter spheres centred on the selected peak voxels.
Figure 2 Activation maps for patients with Huntington's disease compared with those of controls. Patients with Huntington's disease show more task‐related activation compared with controls during performance of the Simon task. Axial slices corresponding to z = –4, 8, 28, 36 and 60 mm are shown.
Figure 3 Regions of interest (ROI) were selected in equivalent left and right hemispheric regions of the lateral prefrontal cortex (LPF and RPF) and the anterior cingulate cortex (LAC and RAC). A functional connectivity model (top right), including all pairwise correlations between the prefrontal ROI, was examined.
Functional connectivity analysis
Mean time courses, averaged across all voxels within each ROI, were calculated for each participant. These time courses were then detrended and mean centred. All scans occurring only during Simon task blocks, excluding the first two scans for each block to allow for the haemodynamic response delay, were concatenated and used for analysis of functional connectivity during the task state. Similarly, all scans occurring during the baseline condition, excluding the first two scans for each block, were concatenated and used to examine functional connectivity during the baseline state. Functional connectivity was then calculated as the correlation between time courses for each pair of prefrontal ROIs (fig 3). These correlations were tested for significance at pcorrected<0.05 (one‐tailed test), using Bonferroni's correction for multiple comparisons. To compare correlations between task and baseline states, and between patients with Huntington's disease and controls, correlation coefficients were transformed to z scores using Fisher's z transformation. z Scores for the differences between task and baseline states, and between patients with Huntington's disease and controls were then calculated.27
Results
Behavioural performance
Behavioural performance on the Simon task for Huntington's disease and control groups was assessed using Mann–Whitney U tests to examine differences between groups and Wilcoxon signed rank tests to compare conditions within groups. Reaction times were significantly slower overall for patients with Huntington's disease (mean (SD) 923 (237) ms) compared with controls (738 (109) ms; z = –3.22, p<0.005), whereas incongruency effects (reaction time differences for incongruent compared with congruent stimuli) were not significantly different between groups (z = –0.81, p = 0.42). The number of errors was also significantly greater for patients with Huntington's disease (mean (SD) 2.1 (3.7)) compared with controls (0.5 (0.7); z = –2.48, p<0.05), whereas incongruency effects (error differences for incongruent compared with congruent stimuli) did not differ between the groups (z = –1.05, p = 0.30). These results have also been included in our other paper (Georgiou‐Karistianis et al, submitted for publication).
Functional connectivity
Significant functional connectivity was found between most regions during both baseline and task states for both Huntington's disease and control groups. Greatest correlations (ie, maximum functional connectivity) were always found between the left and right anterior aingulate cortex (path a, right anterior cingulate cortex (RAC)–left anterior cingulate cortex (LAC); 0.67<r<0.74, pcorrected<0.001). We found no significant correlation between left and right prefrontal cortex regions (path e, left prefrontal cortex (LPF)–right prefrontal cortex (RPF)) for either group (–0.13<r<–0.03, pcorrected>0.05), and correlation between the right anterior cingulate cortex and left prefrontal cortex (path c, RAC–LPF) showed only a low level of correlation during the task for controls and during both conditions for the Huntington's disease group (0.09<r<0.18, pcorrected<0.05). All other connections between anterior cingulate cortex and prefrontal cortex regions showed significant correlation for both conditions and both subject groups (r>0.20, pcorrected<0.01; table 2).
Table 2 Simon task condition correlation coefficients, representing functional connectivity during task scans along each path between left and right prefrontal cortex and left and right anterior cingulate cortex regions.
| Connection | Control | HD | |
|---|---|---|---|
| RAC–LAC | 0.73* | 0.67* | Control > HD * |
| RAC–RPF | 0.52* | 0.37* | Control > HD * |
| RAC–LPF | 0.09* | 0.16* | HD > Control * |
| LAC–RPF | 0.32* | 0.31* | |
| LAC–LPF | 0.25* | 0.21* | Control > HD * |
| LPF–RPF | –0.03 | –0.06 |
HD, Huntington's disease; LAC, left anterior cingulate cortex; LPF, left prefrontal cortex; RAC, right anterior cingulate cortex; RPF, right prefrontal cortex.
*pcorrected<0.05.
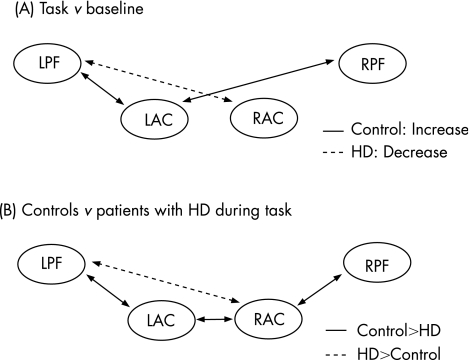
Figure 4 shows the regional changes in functional connectivity between baseline and task conditions, and differences between controls and patients with Huntington's disease. For controls, interhemispheric connections between the anterior cingulate cortex and prefrontal cortex (path c, RAC–LPF; and path d, LAC–RPF) increased significantly during task performance compared with the baseline state (z>4.16, p<0.001). By contrast, patients with Huntington's disease showed no increases in functional connectivity during the task compared with the baseline state, but showed a small decrease in functional connectivity of the LAC to the LPF (z = 3.10, p<0.05).
Figure 4 Prefrontal connections, which show significant differences in functional connectivity between task and baseline states and between controls and patients with Huntington's disease (HD). (A) Controls show significantly increased interhemispheric connectivity during Simon task performance compared with baseline (solid lines), whereas patients with Huntington's disease show significantly reduced connectivity during the task compared with baseline between left anterior cingulate cortex (LAC) and left prefrontal cortex (LPF; dotted lines). (B) During performance of the Simon task, controls show significantly greater functional connectivity compared with patients with HD between several regions (solid lines), whereas patients with HD show greater connectivity than controls between right anterior cingulate cortex (RAC) and LPF. RPF, right prefrontal cortex.
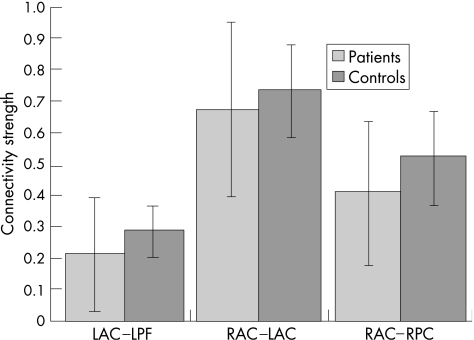
Consequently, when directly comparing functional connectivity during task performance for controls and patients with Huntington's disease (fig 4B), patients with Huntington's disease showed significantly reduced interhemispheric functional connectivity between LAC and RAC regions and reduced intrahemispheric connectivity between the anterior cingulate and prefrontal cortex in both left and right hemispheres (paths a, b, e; z>2.15, p<0.05). Conversely, patients with Huntington's disease showed greater functional connectivity between the RAC and LPF cortex compared with controls (path c; z = 3.28, p<0.05), although the actual degree of functional connectivity along this path was very small (0.09<r<0.16; fig 5).
Figure 5 Plot of mean (SD) of significant functional connectivity during Simon task performance in controls and patients with Huntington's disease. Patients with Huntington's disease show significantly higher variability in functional connectivity than controls.
We further assessed the variability of functional connectivity along the connection paths in which patients with Huntington's disease showed reduced connectivity compared with controls (ie, LPF–LAC, LAC–RAC and RAC–RPF). Variability in functional connectivity during task performance was clearly greater in patients with Huntington's disease than in controls (SDs, fig 5). To explain this variance across patients with Huntington's disease, we further correlated functional connectivity during task performance with clinical measures (age, depression (Beck Depression Inventory), severity of Huntington's disease (CAG index)) and behavioural performance measures (reaction times and errors) using Spearman's rank correlation. Significant correlations were found only for the patients with Huntington's disease along the path LPF–LAC, in which LPF–LAC connectivity was negatively correlated with overall reaction time (r = –0.51, p<0.05) and with overall number of errors (r = –0.60, p<0.01). This indicates that, in patients with Huntington's disease, reduced connectivity between LPF and LAC during task performance was associated with longer reaction times and more frequent errors.
Discussion
During a spatial‐incongruency Simon task, we were able to perform a series of correlations to assess the functional connectivity between prefrontal brain regions—namely, the anterior cingulate and LPF, in patients with Huntington's disease compared with controls. Overall, we found significant functional connectivity, representing significant inter‐regional interactions, between all prefrontal ROIs, except for the connection between LPF and RPF regions. This represents a high degree of connectivity and interaction between regions within the prefrontal cortex.
During performance of the Simon task, compared with baseline, controls showed increased interhemispheric functional connectivity between the anterior cingulate and contralateral lateral prefrontal regions. This represents a greater interhemispheric coupling between the medial and lateral prefrontal regions during performance of the Simon task. Previous studies have similarly shown such dynamic coupling between prefrontal cortical regions associated with cognitive task performance.28,29,30,31 The same pattern, however, was not observed in patients with Huntington's disease who showed no additional coupling, or increased functional connectivity, between prefrontal ROI during task performance. Rather, at an intrahemispheric level, patients with Huntington's disease showed a decrease in correlation between the left anterior cingulate and left lateral prefrontal regions, the level of which was associated with poorer task performance.
Crucially, this decrease in connectivity occurred despite the fact that these same prefrontal regions showed significantly increased BOLD activation during task performance in the patients with Huntington's disease compared with controls. Results suggest that, even though anterior cingulate and lateral prefrontal regions were activated as expected during cognitive task performance, patients with Huntington's disease failed to dynamically increase coupling between prefrontal regions. In fact, patients with Huntington's disease showed a loss in synchrony or interaction between activated regions of the LAC and lateral LPF, in the left hemisphere, which predicted poor task performance.
It is well established that degeneration of brain regions in Huntington's disease affects cortical circuits and leads to loss of cognitive, motor and executive functions.8 The overall loss in synchrony observed during the task suggests that interactions between prefrontal regions are compromised in patients with Huntington's disease. This is consistent with previous knowledge regarding the neuropathology of Huntington's disease and strongly supports the notion of deficits in prefrontal circuitry.32,33
A direct comparison of prefrontal functional connectivity between patients with Huntington's disease and controls during task performance showed that patients are impaired in their functional connectivity between LAC and RAC regions and between the anterior cingulate and lateral prefrontal regions in each hemisphere. Patients did show increased connectivity between the RAC and lateral LPF; however, this connection was relatively weak, with a low correlation coefficient. Overall, therefore, patients show impaired connectivity between prefrontal cortical regions compared with controls. This is despite patients with Huntington's disease showing significantly greater activation within these prefrontal regions compared with controls. Changes in functional activation in patients with Huntington's disease may also influence the coupling between the ROIs; note, however, that disease progression may affect the pattern of activation and connectivity over time. Note also that Paulsen et al10 and Feigin et al20 have found significantly increased activation in similar prefrontal regions in presymptomatic patients with Huntington's disease. It therefore seems that anterior cingulate and lateral prefrontal regions show significant activation but reduced synchrony, representing impaired inter‐regional interaction in Huntington's disease. Further, Ho et al34 similarly showed an increased leftward error in a line bisection task, which correlated with reduced angular gyrus density in Huntington's disease; as their task and the Simon task are visuospatial, this possibly implies reduced left‐hemisphere capacity for visuospatial processing in Huntington's disease. This finding of reduced prefrontal functional connectivity in Huntington's disease parallels similar results in other neurodegenerative disorders such as Alzheimer's and Parkinson's diseases.16,17,29,35
This result is consistent with the loss of synchrony observed in patients with Huntington's disease from baseline to task states. The decreased interhemispheric connectivity may be due to neuronal cell death, as inputs and outputs between certain regions are compromised.8 Behaviorally, interhemispheric abnormalities, or lateralisation deficits, have been previously identified in Huntington's disease.34 It is known that the associative component of the striatum is affected in Huntington's disease, causing disruption to frontostriatal circuitry.6 This could in turn lead to the impaired interaction between prefrontal regions observed herein. Moreover, studies have shown white matter volumetric loss in cortical and subcortical areas in Huntington's disease.36,37,38,39 Such white matter loss in prefrontal regions in Huntington's disease would be expected to affect physical connections between prefrontal cortical regions and consequently impair functional connectivity, as we have observed. Possible white matter loss could also lead to neuronal dysfunction, which may result in impaired connectivity.40
There are two potential limitations of this study. The first is that the congruent and incongruent conditions were not individually assessed. Although this contrast is of potential interest, the correlation between the two tasks was very high, thus not enabling meaningful contrasts between conditions. The second possible limitation relates to the absence of a motor response in the baseline condition. Note, however, that in this study, the contrasts between groups did not involve the baseline condition. Moreover, the baseline condition was only used to show an increase in task‐related connectivity in controls, whereas this effect was not seen in patients with Huntington's disease.
This study has shown that the communication between brain regions in patients with Huntington's disease is significantly weakened during performance of cognitive tasks on two counts. Firstly, the increased connectivity from baseline to task states in controls was not observed in patients with Huntington's disease, and secondly, task‐related functional connectivity overall was impaired in patients with Huntington's disease compared with controls. Collectively, these two findings suggest a loss of synchrony between prefrontal brain regions, suggesting compromised interactions in Huntington's disease. We speculate whether significantly greater levels of activation within prefrontal regions in patients with Huntington's disease (compared with controls) partially compensate for the otherwise compromised interactions between these cortical regions.
Acknowledgements
We thank the Brain Research Institute, Austin Health (Victoria, Australia), and all the participants who were involved in the study for their support.
Abbreviations
BOLD - blood‐oxygen level‐dependent
CAG - cytosine–adenine–guanine
fMRI - functional magnetic resonance imaging
LAC - left anterior cingulate cortex
LPF - left prefrontal cortex
MRI - magnetic resonance image
RAC - right anterior cingulate cortex
ROI - region of interest
RPF - right prefrontal cortex
UHDRS - Unified Huntington's Disease Rating Scale
Footnotes
Funding: This work was supported by funding from Neurosciences Victoria and the National Health and Medical Research Council (Australia), Project Grant (284247) and Fellowships to RC (217025) and GFE.
Competing interests: None.
References
- 1.Harper P, Houlihan G, Jones A.et alHuntington's disease. 2nd edn. Oxford: WB Saunders, 1996
- 2.Georgiou‐Karistianis N, Smith E, Bradshaw J.et al Future directions in research with presymptomatic individuals carrying the gene for Huntington's disease. Neuropsychologia 200359331–338. [DOI] [PubMed] [Google Scholar]
- 3.Haque N, Borghesani P, Isacson O. Therapeutic strategies for Huntington's disease based on a molecular understanding of the disorder. Mol Med 19973175–183. [DOI] [PubMed] [Google Scholar]
- 4.Langbehn D, Brinkman R, Falush D.et al A new model for prediction of the age of onset and penetrance for Huntingtons disease based on cag length. Clin Genet 200465267–277. [DOI] [PubMed] [Google Scholar]
- 5.Joel D, Weiner I. The connections of the primate subthalamic nucleus: indirect pathways and the open‐interconnected scheme of basal ganglia‐thalamocortical circuitry. Brain Res Rev 19972362–78. [DOI] [PubMed] [Google Scholar]
- 6.Alexander G, Delong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 19869357–381. [DOI] [PubMed] [Google Scholar]
- 7.Joel D. Open interconnected model of basal ganglia‐thalamocortical circuitry and its relevance to the clinical syndrome of Huntington's disease. Mov Disord 200116407–423. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence A, Sahakian B, Robbins T. Cognitive functions and corticostriatal circuits: insights from huntingtons disease. Trends Cogn Sci 19982379–388. [DOI] [PubMed] [Google Scholar]
- 9.Saint‐Cyr J A, Taylor A E, Nicholson K. Behavior and the basal ganglia. Adv Neurol 1995651–28. [PubMed] [Google Scholar]
- 10.Paulsen J S, Zimbelman J L, Hinton S C.et al fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington's disease. Am J Neuroradiol 2004251715–1721. [PMC free article] [PubMed] [Google Scholar]
- 11.Clark V P, Lai S, Deckel A W. Altered functional MRI responses in Huntington's disease. Neuroreport 200213703–706. [DOI] [PubMed] [Google Scholar]
- 12.Friston K J. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 1994256–78. [Google Scholar]
- 13.Sun F, Miller M, D'Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. NeuroImage 200421647–658. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz B, Warner B, Fitzer J.et al Investigating the neural basis for functional and effective connectivity. Application to fMRI. Philos Trans R Soc London B Biol Sci 20053601093–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson B, Riddle M, Cohen D.et al Reduced basal ganglia volumes in Tourette's syndrome using three‐dimensional reconstruction techniques from magnetic resonance images. Neurology 199343941–949. [DOI] [PubMed] [Google Scholar]
- 16.Lekeu F, Van der Linden M, Chicherio C.et al Brain correlates of performance in a free/cued recall task with semantic encoding in Alzheimer disease. Alzheimer Dis Assoc Disord 20031735–45. [DOI] [PubMed] [Google Scholar]
- 17.Murphy C, Cerf‐Ducastel B, Calhoun‐Haney R.et al ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer's disease. Chem Senses 200530(Suppl 1)i170–i171. [DOI] [PubMed] [Google Scholar]
- 18.Georgiou N, Bradshaw J L, Phillips J G.et al The Simon effect and attention deficits in Gilles de la Tourette's syndrome and Huntington's disease. Brain 19951181305–1318. [DOI] [PubMed] [Google Scholar]
- 19.Peterson B S, Kane M J, Alexander G M.et al An event‐related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cogn Brain Res 200213427–440. [DOI] [PubMed] [Google Scholar]
- 20.Feigin A, Ghilardi M F, Huang C.et al Preclinical Huntington's disease: compensatory brain responses during learning. Ann Neurol 20065953–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penney J B, Jr, Vonsattel J P, MacDonald M E.et al CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol 199741689–692. [DOI] [PubMed] [Google Scholar]
- 22.Saft C, Andrich J, Meisel N M.et al Assessment of complex movements reflects dysfunction in Huntington's disease. J Neurol 20032501469–1474. [DOI] [PubMed] [Google Scholar]
- 23.Huntington's Disease Study Group Unified Huntington's disease rating scale: reliability and consistency. Mov Disord 199611136–142. [DOI] [PubMed] [Google Scholar]
- 24.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 25.Nelson H, O'Connell A. Dementia. The estimation of pre‐morbid intelligence levels using the New Adult Reading Test. Cortex 197814234–244. [DOI] [PubMed] [Google Scholar]
- 26.Beck C T. The occurrence of depression in women and the effect of the women's movement. J Psychiatr Nurs Ment Health Serv 19791714–16. [PubMed] [Google Scholar]
- 27.Zar J.Biostatistical analysis. Singapore: Pearson Education, 1997
- 28.Egner T, Jamieson G, Gruzelier J. Hypnosis decouples cognitive control from conflict monitoring processes of the frontal lobe. NeuroImage 200527969–978. [DOI] [PubMed] [Google Scholar]
- 29.Grady C L, Furey M L, Pietrini P.et al Altered brain functional connectivity and impaired short‐term memory in Alzheimer's disease. Brain 2001124739–756. [DOI] [PubMed] [Google Scholar]
- 30.Lowe M J, Dzemidzic M, Lurito J T.et al Correlations in low‐frequency BOLD fluctuations reflect cortico‐cortical connections. NeuroImage 200012582–587. [DOI] [PubMed] [Google Scholar]
- 31.Valet M, Sprenger T, Boecker H.et al Distraction modulates connectivity of the cingulo‐frontal cortex and the midbrain during pain‐an fMRI analysis. Pain 2004109399–408. [DOI] [PubMed] [Google Scholar]
- 32.Dursun S M, Burke J G, Andrews H.et al The effects of antipsychotic medication on saccadic eye movement abnormalities in Huntington's disease. Prog Neuropsychopharmacol Biol Psychiatry 200024889–896. [DOI] [PubMed] [Google Scholar]
- 33.Schmidtke K, Manner H, Kaufmann R.et al Cognitive procedural learning in patients with fronto‐striatal lesions. Learn Mem 20029419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho A K, Nestor P J, Williams G B.et al Pseudo‐neglect in Huntington's disease correlates with decreased angular gyrus density. Neuroreport 2004151061–1064. [DOI] [PubMed] [Google Scholar]
- 35.Winterer G, Coppola R, Egan M F.et al Functional and effective frontotemporal connectivity and genetic risk for schizophrenia. Biol Psychiatry 2003541181–1192. [DOI] [PubMed] [Google Scholar]
- 36.Fennema‐Notestine C, Archibald S L, Jacobson M W.et al In vivo evidence of cerebellar atrophy and cerebral white matter loss in Huntington disease. Neurology 200463989–995. [DOI] [PubMed] [Google Scholar]
- 37.Halliday G M, McRitchie D A, Macdonald V.et al Regional specificity of brain atrophy in Huntington's disease. Exp Neurol 1998154663–672. [DOI] [PubMed] [Google Scholar]
- 38.Jernigan T L, Salmon D P, Butters N.et al Cerebral structure on MRI, part II: specific changes in Alzheimer's and Huntington's diseases. Biol Psychiatry 19912968–81. [DOI] [PubMed] [Google Scholar]
- 39.Rosas H D, Koroshetz W J, Chen Y I.et al Evidence for more widespread cerebral pathology in early HD: an MRI‐based morphometric analysis. Neurology 2003601615–1620. [DOI] [PubMed] [Google Scholar]
- 40.Ciarmiello A, Cannella M, Lastoria S.et al Brain white‐matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J Nucl Med 200647215–222. [PubMed] [Google Scholar]