Abstract
We describe a patient found to have acute diffuse and reversible encephalopathy on magnetic resonance imaging (MRI) associated with cholesterol emboli syndrome (CES). The initial MRI showed extensive white matter, basal ganglia and cortical damage without evidence of brain infarction. Dramatic clinical and MRI improvement was observed with corticosteroids. Pathologically, cholesterol crystal emboli were found in the lumen of skin and brain arteries and were associated with varying degrees of inflammation of the arteriole wall. This case suggests that CES may be responsible for extensive, acute and reversible encephalopathy underlined by an inflammation of brain arteries.
Cholesterol emboli syndrome (CES) is a rare but serious complication of arteriosclerosis. It is associated with considerable morbidity and death.1,2 Many organs can be involved, and the clinical manifestations are extremely variable. Key features are acute renal failure, cutaneous lesions such as livedo reticularis and toe necrosis.1 Retinal cholesterol emboli are present in 16% of cases. Neurological manifestations are severe complications of CES and usually consist of multiple small cerebral infarctions.3 We report here a pathologically‐proved case characterised by acute reversible encephalopathy on MRI, a very unusual radiological presentation of CES.
Case report
A 59‐year‐old man was admitted because of acute right hemiparesis and confusion. His medical history was remarkable for diabetes, hypertension, mild chronic renal failure, intermittent claudication and heavy smoking (20 cigarettes daily for 30 years). He had an asymptomatic right internal carotid artery occlusion and had undergone a subclavian‐carotid anostomosis for an asymptomatic left internal carotid artery preocclusive stenosis 3 months earlier. The patient had myocardial infarction 2 months before admission. His coronarography showed a two‐vessel coronary artery disease, but angioplasty was not carried out. His creatinine level then was 209 μmol/l.
The patient had bilateral leg pain 2 weeks before admission. Leg arteriography was performed, which disclosed mild stenosis of both common iliac arteries. Intravenous heparin was started. The following day, he suddenly became stuporous. He was referred to the tenon stroke unit. Neurological examination showed right hemiparesis, aphasia and confusion with fluctuating consciousness. Systolic blood pressure ranged from 170 to 134 mm Hg and diastolic blood pressure from 85 to 57 mm Hg. Physical examination showed toe necrosis and livedo reticularis. Ophthalmological examination showed grade I hypertensive retinopathy without retinal emboli. Laboratory investigations disclosed mild leucocytosis (10 800/mm3) with eosinophilia (920/mm3) and raised C reactive protein level (31 mg/l), and an erythrocyte sedimentation rate of 76 mm/h. Plasma creatinine had risen to 351 μmol/l. Heparin was discontinued.
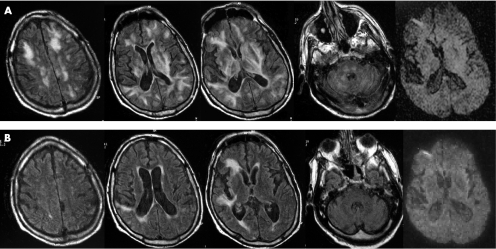
MRI showed extensive bilateral hypersignals on fluid‐attenuated inversion recovery weighted images suggestive of vasogenic oedema predominantly involving the white matter of the cerebral hemispheres, but also affecting the basal ganglia, cerebral cortex, brainstem, and cerebellum (fig 1A). There was no evidence of cytotoxic oedema or cerebral infarct on diffusion‐weighted images (DWI). No enhancement was seen after gadolinium injection. Transoesophageal echocardiography showed protruding atheromatous lesions of the aortic arch and descending aorta. Biopsy specimen of the skin showed cholesterol emboli and inflammatory infiltrates of the artery walls. Repeated electroencephalograms did not show evidence for epileptic seizures.
Figure 1 (A) Initial MRI findings. Fluid attenuated inversion recovery (FLAIR) sequence image showing diffuse white matter hyperintensity at both infra and supratentorial levels and in the basal ganglia. Hyperintensity extends from the periventricular white matter to the cortex. Diffusion‐weighted images (DWI)‐weighted MRI was normal. (B) MRI 3 weeks later. FLAIR sequence image showing dramatic improvement of the abnormalities but two areas of hyperintense signals in the right frontal region are shown without abnormalities on DWI‐weighted MRI.
After 3 days, the patient experienced worsening renal function and a severe inhalation pneumonia requiring intubation. Medical treatment included corticosteroids, antibiotics, clopidogrel, statins and haemodialysis. Treatment with corticosteroids consisted of intravenous methylprednisolone (80 mg/day for 5 days) followed by oral prednisone (0.5 mg/kg/day). The respiratory and renal functions improved gradually and extubation was performed. His aphasia and right hemiplegia recovered slowly. The patient was fully vigilant, oriented and was left with no neurological sequelae 2 weeks later. Follow‐up MRI performed 3 weeks after the first MRI showed near‐complete resolution of the abnormalities, with persistence of two cortical hypersignals consistent with right middle cerebral artery infarcts (fig 1B). The subsequent course was complicated by a septic shock secondary to pneumonia. The patient died 2 days later despite intensive care.
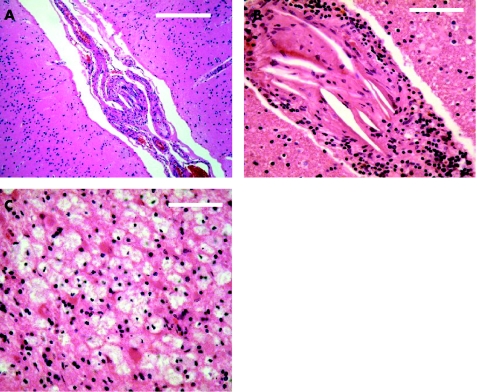
The autopsy showed bronchopneumonia and severe aortic atherosclerosis. Brain histopathology showed numerous scattered small cortical and subcortical infarcts in the territory of the right middle cerebral artery. The characteristic shape of cholesterol crystals was identified within the connective tissue that occluded the lumen of small leptomeningeal and perforating brain arteries, confirming cholesterol embolism (fig 2). Small reactive lymphocytes infiltrated the walls of some arteries, but no fibrinoid necrosis was identified.
Figure 2 Brain histopathology. Small leptomeningeal (A) and perforating brain (B) arteries containing numerous cholesterol clefts and focal accumulation of lymphocytes in the adventitia. Lipid‐laden macrophages and reactive astrocytes in a small subcortical infarct (C). Scale bars: (A) 100 μm, (B) 50 μm, (C) 50 μm.Hematoxylin‐eosin stain.
Discussion
In this case, we report a histopathologically‐proved cholesterol embolism syndrome remarkable for the presence of severe and reversible acute encephalopathy on MRI. Brain injury consisted of extensive leucoencephalopathy associated with basal ganglia and cortex damage. Except for the neuroradiological aspect, this polyvascular patient showed typical features of severe CES with multivisceral ischaemia leading to rapidly progressive renal failure, cutaneous symptoms, declining general status, biological inflammatory syndrome and eosinophilia. Even though contrast‐induced nephropathy might have contributed to the degradation of his renal function, renal failure was attributed mainly to CES because of its concomitant occurrence with other systemic signs of CES. In our case, as in other classical cases, a source of cholesterol emboli was found with severe atherosclerotic lesions of the aortic arch, and predisposing factors for CES were: a recent vascular surgery by subclavian‐carotid anastomosis, repeated arterial catheterisms and inappropriate anticoagulation. Neurological findings were characterised by the association of encephalopathy and focal neurological signs.
Cholesterol emboli to the brain are known to occlude small arteries with a diameter of 17–585 μm and lead to multiple small infarctions.1,4,5 In the largest reported series of cholesterol emboli to the brain, clinical and radiological characteristics were reviewed in 29 autopsy‐proved cases.3 Encephalopathy, mostly severe, was the predominant finding on examination in 75% of patients. Focal neurological deficits were found in 42% of patients, isolated in 1 of 3 patients and associated with encephalopathy in the others. Brain imaging, computed tomographic scan or MRI was normal in 35% of patients. In the other cases, cerebral infarctions were shown, most often multiple or in the border zone distribution.
To our knowledge, reversible encephalopathy on MRI has never been reported in the setting of CES. The MRI of our patient showed extensive hemispheric white and grey matter abnormalities consistent with vasogenic oedema, and a subsequent MRI showed near complete resolution of these abnormalities. Post‐mortem pathological examination confirmed cholesterol emboli to the brain, suggesting that CES can cause acute and reversible radiological encephalopathy. The mechanism of this reversible encephalopathy associated with CES is unknown. Although the follow‐up MRI and neuropathological examination showed brain infarctions, the initial MRI showed no evidence of cytotoxic oedema, excluding the hypothesis that direct ischaemic damage due to arteriolar occlusion was responsible for the patient's encephalopathy. Surprisingly, initial or follow‐up MRI and brain pathology did not show a relevant infarct that would explain the focal neurological deficit which resolved in just 2–3 weeks.
The radiological pattern of our case mimicked by its rapid favourable course the reversible posterior leucoencephalopathy syndrome (RPLS), but RPLS has never been described with CES. Our patient had no abrupt increase in blood pressure and no other predisposing condition for RPLS. Additionally, the leucoencephalopathy was not predominantly posterior as observed in RPLS. However, our case may share a similar pathophysiological mechanism with RPLS. Hence, cholesterol emboli in the artery lumen may alter vascular reactivity and the blood–brain barrier permeability, leading to a reversible leucoencephalopathy.6
Another likely explanation of this reversible encephalopathy is an inflammatory mechanism associated with CES. Inflammatory infiltrates were found in our case in the wall of skin and brain arterioles. The presence of cholesterol crystals within the vascular lumen may trigger a localised inflammatory and endothelial vascular reaction.1 Some immunosuppressive drugs, such as corticosteroids, reduce the inflammatory reaction at the site of embolisation in distal arteries, and have been associated with improvement in a few severe cases.2,7,8 Our patient's course was remarkable for improvement of his neurological and renal status with corticosteroids. This dramatic resolution of white and grey matter abnormalities is suggestive of an underlying cerebral inflammatory disorder.
Abbreviations
CES - cholesterol emboli syndrome, DWI, diffusion‐weighted imaging
Footnotes
Competing interests: None.
References
- 1.Fine M J, Kapoor W, Falanga V. Cholesterol crystal embolization: a review of 221 cases in the English literature. Angiology 198738769–784. [DOI] [PubMed] [Google Scholar]
- 2.Belenfant X, Meyrier A, Jacquot C. Supportive treatment improves survival in multivisceral cholesterol crystal embolism. Am J Kidney Dis 199933840–850. [DOI] [PubMed] [Google Scholar]
- 3.Ezzeddine M A, Primavera J M, Rosand J.et al Clinical characteristics of pathologically proved cholesterol emboli to the brain. Neurology 2000541681–1683. [DOI] [PubMed] [Google Scholar]
- 4.Beal M F, Williams R S, Richardson E P., Jret al Cholesterol embolism as a cause of transient ischemic attacks and cerebral infarction. Neurology 198131860–865. [DOI] [PubMed] [Google Scholar]
- 5.Laloux P, Brucher J M. Lacunar infarctions due to cholesterol emboli. Stroke 1991221440–1444. [DOI] [PubMed] [Google Scholar]
- 6.Hinchey J, Chaves C, Appignani B.et al A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996334494–500. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa M, Kawashima S, Shikano M.et al The evaluation of corticosteroid therapy in conjunction with plasma exchange in the treatment of renal cholesterol embolic disease. A report of 5 cases. Am J Nephrol 200020263–267. [DOI] [PubMed] [Google Scholar]
- 8.Mann S J, Sos T A. Treatment of atheroembolization with corticosteroids. Am J Hypertens 200114831–834. [DOI] [PubMed] [Google Scholar]