Abstract
Anti‐glutamic acid decarboxylase antibody is associated with the development of progressive cerebellar ataxia and slowly progressive insulin‐dependent diabetes mellitus. Previously, the neurophysiological characteristics of IgG in the cerebrospinal fluid of a patient with anti‐glutamic acid decarboxylase antibody‐associated progressive cerebellar ataxia and slowly progressive insulin‐dependent diabetes mellitus were reported. Using a voltage‐gated whole‐cell recording technique, it was observed that the IgG in the cerebrospinal fluid of the patient selectively suppressed the inhibitory postsynaptic currents in the Purkinje cells. The patient died from aspiration pneumonia. Postmortem examination showed almost complete depletion of the Purkinje cells with Bergmann gliosis. Therefore, the main cause of cerebellar ataxia observed in this case may be attributed to the near‐complete depletion of the Purkinje cells. In this paper, the pathomechanisms underlying Purkinje cell damage are discussed.
Glutamic acid decarboxylase (GAD) is a catalytic enzyme that converts glutamic acid to γ‐aminobutyric acid, a major inhibitory neurotransmitter. A disease group that is characterised by the presence of a circulating autoantibody against GAD (anti‐GAD antibody) includes the following: slowly progressive insulin‐dependent diabetes mellitus (SPIDDM), stiff‐person syndrome (SPS) and progressive cerebellar ataxia (PCA).1,2,3 Anti‐GAD antibody is one of the serological diagnostic markers of these diseases. Honnorat et al4 reported a significant link between the anti‐GAD antibody and cerebellar ataxia after screening 9000 serum samples. In addition, autoimmune mechanisms against GAD are presumed to be the causative agents of these diseases.5 Here, we report the autopsy findings of PCA with anti‐GAD antibody and discuss the pathomechanism of this rare disease.
Case report
We previously reported part of the clinical course of a patient with PCA and SPIDDM, and showed the neurophysiological characteristics of IgG in the cerebrospinal fluid.6 In September 1996, a 66‐year‐old woman developed cerebellar ataxia of the limbs and trunk. In April 1997, she had sudden onset of hyperglycaemia, and was subsequently diagnosed with anti‐GAD‐associated SPIDDM. In May 1997, she was bedridden due to severe cerebellar ataxia; other symptoms such as extrapyramidal or pyramidal tracts were not observed. The patient was diagnosed with anti‐GAD antibody‐associated PCA, and received four rounds of plasma exchange and immunosuppressive treatment. After treatment, the patient showed slight improvement in cerebellar ataxia.
In December 2000, the patient experienced painful spasms and rigidity in the trunk that mimicked symptoms of SPS. Diazepam and baclofen were effective in ameliorating the severe pain associated with the spasms and rigidity. The painful spasms subsided spontaneously within 2 months. The patient died of aspiration pneumonia in October 2001.
During the 5‐year clinical course, repeated neuroradiological examinations showed no significant cerebellar atrophy. Using a voltage‐gated whole‐cell recording technique, we observed that the IgG in the cerebrospinal fluid of the patient, selectively suppressed the inhibitory postsynaptic currents in the Purkinje cells.6,7
Postmortem examination
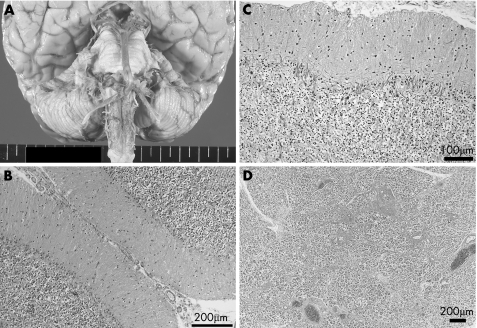
Postmortem examination was performed 22 h after death. The brain weighed 1150 g. The brain and the entire spinal cord were fixed in formalin and prepared for a morphological examination. Macroscopically, there was no atrophy of the cerebrum, brain stem, cerebellum (fig 1A) and spinal cord. The representative areas were examined by routine and immunohistochemical staining, as reported previously.8 In short, 6‐μm thick serial sections were stained with haematoxylin and eosin, Klüver–Barrera and Bodian silver staining. For the immunohistochemical study, 6‐μm dewaxed and microwave‐irradiated sections were stained using a Ventana 20NX automatic stainer (Ventana, Tucson, Arizona, USA). Microscopical examination showed almost complete depletion of the Purkinje cells and diffuse proliferation of the Bergmann glia (fig 1B). The number of remaining Purkinje cells was no more than one per cerebellar folium. Bodian staining showed multiple empty baskets (fig 1C). There was no specific inflammatory response, and the other structures of the central nervous system, including the cerebral cortex, white matter, basal ganglia, brain stem and spinal cord, did not show marked pathological changes. The pancreas showed a definite and marked decrease in the islets in the tail (fig 1D), and lymphocytic infiltration in the islets situated in the pancreatic body.
Figure 1 (A) Macroscopic appearance of the brain stem and cerebellum. There are no atrophic changes in the cerebellum and brain stem. (B) Haematoxylin and eosin staining of the cerebellar cortex. There is severe depletion of Purkinje cells and proliferation of Bergmann glia. (C) Bodian staining of the cerebellar cortex. Multiple empty baskets can be observed. (D) Pancreatic tail (haematoxylin and eosin staining). There is a selective decrease in the pancreatic islets.
Discussion
The selective loss of both Purkinje cells and pancreatic islets was a characteristic finding in this case. The selective degeneration of the Purkinje cells partially mimics the pathological changes observed in paraneoplastic cerebellar ataxia associated with anti‐mGluR1 or anti‐Yo antibody; however, the exclusive pathological changes related to the Purkinje cells constitute a unique feature of this case.9,10 On the other hand, the lymphocytic infiltration in the pancreas and the selective decrease in the pancreatic islets corresponded with the pathological findings of autoimmune insulin‐dependent diabetes mellitus.11 Therefore, the main causes of cerebellar ataxia and diabetes mellitus seem to be related to the depletion of the Purkinje cells and the decrease in the pancreatic islets, respectively. To our knowledge, this is the first autopsy report of PCA associated with anti‐GAD antibody.
Immunohistochemical staining using anti‐GAD and anti‐calbindin antibodies failed to react with the patient's specimen; this indicated a complete loss of antigenicity in the patient's specimen, due to postmortem delay and excessive fixation. Therefore, it became difficult to analyse the morphological changes in the other GAD‐containing neurones, such as the cerebellar basket cells and the spinal Renshaw cells. However, the existence of multiple empty baskets suggested that, in contrast to the Purkinje cells that were lost, the basket cells were relatively preserved.12
We inferred two possible pathomechanisms to explain the Purkinje cell damage: indirect and direct immune‐mediated mechanisms. The indirect mechanism might be associated with excitotoxicity of the Purkinje cells by the selective suppression of inhibitory postsynaptic currents and the attenuation of inhibition of excitatory postsynaptic currents by the anti‐GAD antibody.6,7,13 The direct mechanism might be mediated by cytotoxic reactions against the Purkinje cells caused by the invading leucocytes, as observed in the pancreatic islets. However, it is presently unclear whether the mechanisms that are more likely to have caused the Purkinje cell damage are indirectly or directly immune‐mediated.
The patient experienced painful muscle spasms that mimic symptoms of SPS. The muscle spasms observed in SPS are considered to occur as a result of the dysfunction of the Renshaw cells that are γ‐aminobutyric acid inhibitory interneurones in the spinal cord.14 Various pathological changes are observed in the spinal cord of patients with SPS; however, lymphocytic cuffing and a decrease in the number of anterior horn neurones are considered to be representative of SPS.15 In contrast, the pathological changes observed in our patient were unremarkable; this suggests that the Renshaw cells were not severely damaged. This may explain the transient nature of the muscular spasms in this case.
Based on the quantitative analysis of the brain autopsy of a patient with SPS and without cerebellar ataxia, Warich‐Kirches et al16 reported diminished cell density of the inhibitory neurones in the cerebellar cortex. Combining their case results with ours might show the phenotypic overlap of the anti‐GAD autoimmunity‐associated neurological diseases.
Abbreviations
GAD - glutamic acid decarboxylase
PCA - progressive cerebellar ataxia
SPIDDM - slowly progressive insulin‐dependent diabetes mellitus
SPS - stiff‐person syndrome
Footnotes
Competing interests: None declared.
Informed consent was obtained from the family of the patient for the publication of her details in this paper.
References
- 1.Seissler J, Amann J, Mauch L.et al Prevalence of autoantibodies to the 65‐ and 67‐kD isoforms of glutamate decarboxylase in insulin‐dependent diabetes mellitus. J Clin Invest 1993921394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saiz A, Arpa J, Sagasta A.et al Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late‐onset insulin‐dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology 1997491026–1030. [DOI] [PubMed] [Google Scholar]
- 3.Vianello M, Tavolato B, Giometto B. Glutamic acid decarboxylase autoantibodies and neurological disorders. Neurol Sci 200223145–151. [DOI] [PubMed] [Google Scholar]
- 4.Honnorat J, Saiz A, Giometto B.et al Cerebellar ataxia with anti‐glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol 200158225–230. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas M C, Li M, Fujii M.et al Stiff person syndrome: quantification, specificity, and intrathecal synthesis of GAD65 antibodies. Neurology 200157780–784. [DOI] [PubMed] [Google Scholar]
- 6.Ishida K, Mitoma H, Song S Y.et al Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol 199946263–267. [PubMed] [Google Scholar]
- 7.Mitoma H, Song S Y, Ishida K.et al Presynaptic impairment of cerebellar inhibitory synapses by an autoantibody to glutamate decarboxylase. J Neurol Sci 200017540–44. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Suzuki K, Nanba E.et al Niemann‐Pick type C disease: accelerated neurofibrillary tangle formation and amyloid beta deposition associated with ApoE e4 homozygosity. Ann Neurol 200252351–355. [DOI] [PubMed] [Google Scholar]
- 9.Coesmans M, Smitt P A, Linden D J.et al Mechanisms underlying cerebellar motor deficits due to mGluR1‐autoantibodies. Ann Neurol 200353325–336. [DOI] [PubMed] [Google Scholar]
- 10.Verschuuren J, Chuang L, Rosenblum M K.et al Inflammatory infiltrates and complete absence of Purkinje cells in anti‐Yo‐associated paraneoplastic cerebellar degeneration. Acta Neuropathol (Berl) 199691519–525. [DOI] [PubMed] [Google Scholar]
- 11.Nakanishi K, Kobayashi T, Miyashita H.et al Relationships among residual beta cells, exocrine pancreas, and islet cell antibodies in insulin‐dependent diabetes mellitus. Metabolism 199342196–203. [DOI] [PubMed] [Google Scholar]
- 12.Gatti R A, Vinters H V. Cerebellar pathology in ataxia‐telangiectasia: the significance of basket cells. Kroc Found Ser 198519225–232. [PubMed] [Google Scholar]
- 13.Mitoma H, Ishida K, Shizuka‐Ikeda M.et al Dual impairment of GABAA‐ and GABAB‐receptor‐mediated synaptic responses by autoantibodies to glutamic acid decarboxylase. J Neurol Sci 200320851–56. [DOI] [PubMed] [Google Scholar]
- 14.Meinck H M, Ricker K, Hulser P J.et al Stiff man syndrome: clinical and laboratory findings in eight patients. J Neurol 1994241157–166. [DOI] [PubMed] [Google Scholar]
- 15.Warren J D, Scott G, Blumbergs P C.et al Pathological evidence of encephalomyelitis in the stiff man syndrome with anti‐GAD antibodies. J Clin Neurosci 20029328–329. [DOI] [PubMed] [Google Scholar]
- 16.Warich‐Kirches M, Von Bossanyi P, Treuheit T.et al Stiff‐man syndrome: possible autoimmune etiology targeted against GABA‐ergic cells. Clin Neuropathol 199716214–219. [PubMed] [Google Scholar]