Rasmussen encephalitis is a rare unihemispheric inflammatory disease of the brain that leads to intractable seizures, cognitive decline and progressive neurological deficits associated with the affected hemisphere. It predominantly affects children, with the onset in adults having a milder course. Immunotherapy has been suggested to improve the outcome of Rasmussen encephalitis.1,2
Case report
In November 2000, a left‐handed 37‐year‐old woman experienced a head trauma with brief loss of consciousness. Shortly after, she had mild clumsiness of her right leg, which went on for the next 2 years. No magnetic resonance imaging (MRI) study was performed at that time. Her family also noticed a change in her character (all of which was retrospectively interpreted as the “prodromal stage” of Rasmussen encephalitis2).
In February 2002, the patient started having epilepsia partialis continua (EPC) of her right hand (later on interpreted as an onset of the “acute stage” of Rasmussen encephalitis2). Apart from EPC and impaired motor function of her right leg and hand (due to the EPC), the neurological examination and electroencephalogram were normal at this time. Cerebrospinal fluid contained 10 cells/μl, had a normal protein level and showed oligoclonal bands. Microbiological studies showed no sign of an infectious agent. MRI of the brain showed a mild left temporal atrophy. A steroid pulse treatment was given. However, the motor deficit progressed, accentuated in the right hand and leg, with central sensory deficit.
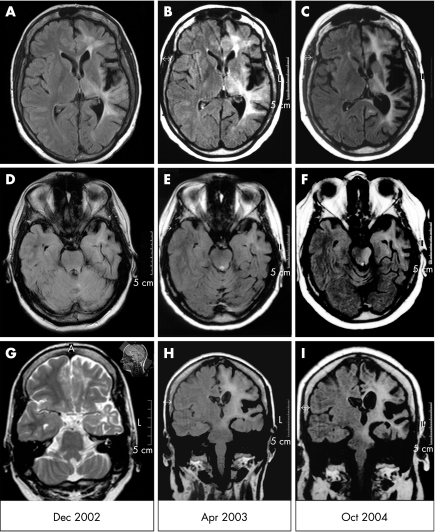
In December 2002, Jacksonian motor seizures of the patient's right hemibody started evolving from the EPC. Brain MRI showed left‐sided supratentorial atrophy (most pronounced around the Sylvian fissure) and increased fluid‐attenuated inversion recovery or T2 signal of the white matter. The brain stem, however, was neither atrophic nor did it show an increased signal (fig 1A, D, G).
Figure 1 Serial brain fluid‐attenuated inversion recovery‐magnetic resonance imaging (FLAIR‐MRI) scans in the patient described here. (A–C) Axial sections including the Sylvian fissure; (D–F) axial sections through the upper brain stem; (G–I) coronal sections including the pons. For the time course, see the dates on the bottom of the figure. (G) As no coronal FLAIR or T2 images from December 2002 exist, this unusually angulated T2 section showing the pons (for orientation of slices, see the small image in the upper right corner of (G)) from July 2002 is used as a substitute. A slight atrophy of the left cerebellar hemisphere is also seen.
In April 2003, the patient presented to our department (Department of Epileptology, University of Bonn, Bonn, Germany). MRI scans showed progression of hemiatrophy of the left hemisphere and involvement of the left mesencephalon (fig 1B, E, H). Biopsy specimens of the brain biopsy, obtained from the left superior frontal gyrus, showed perivascular and parenchymatous CD3+ CD8+ T lymphocytes (partly in close apposition to neurones), microglial activation and astrogliosis. The patient received a total of 1.2 g intravenous immunoglobulins (IvIg) per kilogram body weight.
On discharge, the patient had a 4/5 right hemiparesis with hypoesthesia. Further, monthly courses of 0.4 g IvIg/kg were recommended.3 The patient's compulsory health insurance, however, refused to cover the costs for this kind of treatment. Three months later, the patient's hemiparesis had markedly progressed (arm, 2–3/5; leg, 4/5). Despite reinstitution of monthly IvIg by inpatient treatments in our department, the patient was hemiplegic by October 2003 and became seizure free at about the same time (onset of the “residual stage”2). Fortunately, the patient's language abilities were preserved, obviously owing to atypical dominance (functional MRI scan disclosed bilateral, predominantly right‐sided activation of frontotemporal regions during language tasks). IvIg treatment was stopped.
In April 2004, the patient was admitted because of swallowing and speech problems. On cranial nerve examination, she had a newly observed deviation of the uvula to the left side and reduced soft‐palate elevation; gag and cough reflexes were normal, and speech showed signs of a flaccid dysarthria. Neither oculomotor abnormalities nor other signs of upper brain stem were affected. No cerebellar signs on the unaffected side were noted. The MRI scan showed an ongoing progression of the supratentorial left‐sided hemiatrophy and an increase in signal extending subcortically to the left mesencephalon and pons, without contrast enhancement. This strictly unilateral signal increase in the left pons was newly observed (fig 1C, F, I). A high‐dose long‐term oral steroid treatment was started. One year later, swallowing and speech problems as well as the palatal velus paresis had resolved. MRI was unchanged. The patient is now 41 years old and remains seizure free.
Discussion
To the best of our knowledge, this is the first published biopsy‐proven case of an adult‐onset Rasmussen encephalitis with magnetic resonance–tomographically demonstrated affection of the brain stem. The patient presented here exhibited a large increase in the fluid‐attenuated inversion recovery signal, suggesting active inflammation or strong astrogliosis of the left upper brain stem in continuity with the supratentorial lesion, and clearly delineated from the right side. This is remarkable, as the characteristic and puzzling property of Rasmussen encephalitis of respecting the midline of the cerebral hemispheres is also observed here within the tight space of the brain stem. It cannot, however, be ruled out that Wallerian degeneration secondary to the supratentorial lesion contributes to this MRI presentation. The clinical improvement after resuming the immunosuppressive treatment also supports an ongoing active inflammatory process, even through the persistence of the radiological lesion. The relative mildness of the brain stem symptoms as well as the lack of topographical concordance should be noted, but does not contribute to the aetiological clarification. A possible explanation for this lower cranial nerve symptomatology without evident medullar involvement may be a supranuclear affection of the corticobulbar pathways.4
Infratentorial involvement in the form of cerebellar atrophy has previously been observed in childhood‐onset cases, partly ipsilaterally and partly contralaterally (in the sense of a “cerebellar diaschisis”), however, without reported clinical correlates.5 In our patient, mild cerebellar hemiatrophy ipsilateral to the cerebral hemiatrophy without clinical correlates was observed.
To conclude, this case is an example of ongoing damage to the central nervous system, including the brain stem, even during the “residual disease stage”, a feature indicating a late relapse of disease activity. This is particularly surprising given the fact that patients with adult‐onset Rasmussen encephalitis usually experience a rather mild course and a relatively good long‐term outcome.2 This case may broaden the clinical and neuroradiological spectrum of possible courses of Rasmussen encephalitis.
Footnotes
Competing interests: None.
References
- 1.Bien C G, Granata T, Antozzi C.et al Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 2005128454–471. [DOI] [PubMed] [Google Scholar]
- 2.Bien C G, Widman G, Urbach H.et al The natural history of Rasmussen's encephalitis. Brain 20021251751–1759. [DOI] [PubMed] [Google Scholar]
- 3.Hart Y M, Cortez M, Andermann F.et al Medical treatment of Rasmussen's syndrome (chronic encephalitis and epilepsy): effect of high‐dose steroids or immunoglobulins in 19 patients. Neurology 1994441030–1036. [DOI] [PubMed] [Google Scholar]
- 4.Iwata M. Unilateral palatal paralysis caused by lesion in the corticobulbar tract. Arch Neurol 198441782–784. [DOI] [PubMed] [Google Scholar]
- 5.Chiapparini L, Granata T, Farina L.et al Diagnostic imaging in 13 cases of Rasmussen's encephalitis: can early MRI suggest the diagnosis? Neuroradiology 200345171–183. [DOI] [PubMed] [Google Scholar]