Hashimoto's encephalopathy is an autoimmune encephalopathy that came to be regarded as a new clinical entity distinct from myxo‐oedema encephalopathy, associated with Hashimoto's thyroiditis.1,2
Hashimoto's encephalopathy has a wide clinical spectrum with various neuropsychiatric features. The detection of antithyroid antibodies in patient sera is helpful but not sufficient for the diagnosis of Hashimoto's encephalopathy because of the high prevalence of antibodies in the normal population.
Recently, we reported serum autoantibodies against the amino (NH2) terminal region of α enolase (NAE) as a useful diagnostic marker of Hashimoto's encephalopathy.3
We describe here a patient with Hashimoto's encephalopathy, who presented with progressive cerebellar ataxia with mild abnormality on electroencephalography (EEG) and showed marked improvement after steroid administration. The patient was diagnosed as having Hashimoto's encephalopathy owing to the presence of the anti‐NAE antibodies as well as antithyroid antibodies in the serum.
A 41‐year‐old woman, who had a normal dietary history, became aware of a slight unsteadiness while walking and mild dysarthria in December 2003. She had no familial history of neurological disorders or episodes of seizures. The symptoms gradually worsened, and she was admitted to the University of Fukui hospital in September 2004 because she could not stand or walk without support.
Neurological examinations showed severe gait ataxia, slurred speech and dysmetria on finger‐to‐nose and heel‐to‐knee manoeuvres. Cognitive functions and intellectual performance were normal. Ocular movement was full and smooth and without nystagmus. Deep tendon reflexes were normal and without any pathological reflex. No apparent paresis abnormal sensations including deep sensations, extrapyramidal signs or autonomic dysfunctions were found.
Magnetic resonance imaging of the brain did not detect any atrophy of the cerebellum or any abnormal signal. EEG showed diffuse slow‐wave activities (7–8 Hz) without any epileptic discharge. Analysis of the cerebrospinal fluid did not show any pleocytosis or increases in protein (15 mg/dl) and immunoglobulin (Ig)G (1.2 mg/dl) levels. Peripheral blood cell counts, electrolytes, liver and kidney functions, and levels of lactate, ammonia, vitamins B1, B12 and E were all normal. Serological markers specific for collagen diseases such as antinuclear, anti‐DNA, anti‐Sm, anti‐RNP, anti‐SSA, anti‐SSB, anti‐glutamic acid decarboxylase (GAD) antibodies, c‐antineutrophil cytoplasmic antibodies and myeloperoxidase‐antineutrophil cytoplasmic antibodies were either negative or in the normal range. Titters of antibodies against Herpes simplex, Varicella‐zoster, Epstein‐Barrvirus, cytomegalovirus and echo viruses were not raised in the serum or in the cerebrospinal fluid. Anti‐Hu, anti‐Yo and anti‐Ri antibodies were also negative. Tumour markers such as carcinoembryonic antigen, cancer antigen (CA)19‐9 and CA125 were normal. Investigation for malignancy did not detect any sign of malignant disease. Gene analyses did not detect any mutation for hereditary spinocerebellar ataxia (SCA1, SCA6, Machado–Joseph disease and dentate‐rubro‐pallido‐luysian atrophy) or mitochondrial diseases (MELAS and MERRF).
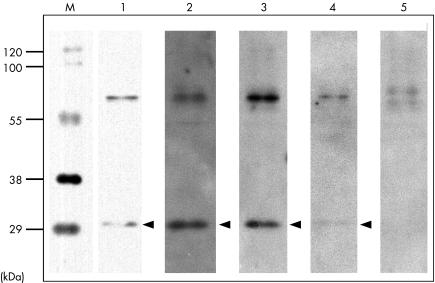
Investigation for thyroid showed euthyroidism (thyroid‐stimulating hormone (TSH), 1.7 μU/ml; normal, 0.4–4.0 μU/ml, free T3, 3.4 pg/ml; normal, 2.4–4.3 pg/ml, free T4, 0.9 ng/dl; normal, 0.9–1.8 ng/dl) and raised levels of antithyroid peroxidase (50.0 U/ml; normal, 0–0.3 U/ml) and antithyroglobulin antibodies (4.9 U/ml; normal, 0–0.3 U/ml). Anti‐NAE antibodies in the serum—a diagnostic marker of Hashimoto's encephalopathy—were strongly positive before steroid treatment, then changed to a weak signal on an immunoblot, after treatment, determined using its recombinant protein expressed in human cultured cells, as described previously (fig 1).3 The ethics committee of the University of Fukui approved this research. Written permission was obtained from the patient.
Figure 1 Immunoblot of a recombinant amino (NH2) terminal region of α enolase (NAE) with sera from an ataxic patient with Hashimoto's encephalopathy. M, molecular weight marker; lane 1, recombinant NAE protein; lane 2, serum from a positive control (patient with Hashimoto's encephalopathy, lanes 3, serum from the present case before steroid treatment; lane 4, serum from the present case after steroid treatment; lane 5, serum from a normal control. Arrowheads indicate the position of NAE. Sera were diluted 300‐fold. A strong signal against the NAE was detected in serum from the present case, and became weaker after steroid administration.
After intravenous administration of high‐dose methylprednisolone (1 g/day) for 3 days, followed by oral administration of prednisolone (30 mg/day), the ataxia improved markedly, and the patient was able to walk unaided 3 weeks after the start of the treatment. The ataxia almost disappeared after continuous treatment for 3 months. Severity of ataxia was evaluated by the size of the estimated area when the patient stood on a stabilimeter. Estimated areas became markedly smaller on the stabilimeter after steroid treatment: pretreatment area, 13.9×8.4 = 116.76 cm2, post‐treatment area, 4.8×3.4 = 16.32 cm2. Slow‐wave activities on EEG partially improved after the treatment.
Pure cerebellar ataxia can be caused by various reagents (alcohol or drugs), or may accompany vitamin deficiencies, viral infections, collagen diseases, autoimmune conditions (anti‐GAD antibodies), spinocerebellar degenerations, neoplasm or mitochondrial diseases with or without cerebellar atrophy. Although anti‐GAD antibodies were detected in the sera from patients with ataxia and type 1 diabetes,4 the antibodies were not detected in our patient. Other possible causes of ataxia were excluded by the clinical history, and laboratory and radiological findings in the present case. Although hypothyroidism is another well‐known cause of ataxia, she had normal thyroid functions.
Patients with Hashimoto's encephalopathy present with a variety of neuropsychiatric symptoms or signs such as hypertonia, tremors, myoclonus, choreoathetosis, seizures, dementia, psychiatric symptoms and strokes.1,2 Ataxia is also reported in some patients with Hashimoto's encephalopathy.2,5 Compared with these reported cases of Hashimoto's encephalopathy with ataxia, the clinical findings in our patient are unique: (1) absence of neurological findings other than ataxia except for mild EEG abnormality and (2) the insidious onset and slow progression of ataxia. Other reported cases of ataxia showed acute or subacute progressions accompanied by other neurological symptoms or signs.2,5 Selim and Drachman5 reported six patients with cerebellar ataxia associated with Hashimoto's thyroiditis, in most of whom cerebellar atrophy was shown on magnetic resonance imaging. One of their reported cases was treated by intravenous immunoglobulin IgG, and ataxia partially improved.5 Although responsiveness to intravenous immunoglobulin suggested autoimmune mechanisms in the pathogenesis of ataxia in this patient, it remains uncertain whether or not ataxia and cerebellar atrophy were aetiologically associated with Hashimoto's thyroiditis. By contrast, our patient with progressive ataxia had a positive serological diagnostic marker, anti‐NAE antibodies and showed an excellent response to steroid treatment, leading to a diagnosis of Hashimoto's encephalopathy.
In conclusion, this report suggests that a diagnosis of Hashimoto's encephalopathy is warranted in patients with progressive pure ataxia and anti‐NAE antibodies are a useful serological marker of diagnosis. Moreover, Hashimoto's encephalopathy should be included in a differential diagnosis of a treatable ataxia.
Acknowledgements
We thank Tomomi Kame for technical assistance.
Footnotes
Funding: This work was supported in part by a Neuroimmunological Disease Committee grant from the Ministry of Health, Labor and Welfare of Japan (to MY).
Competing interests: None declared.
References
- 1.Shaw P J, Walls T J, Newman P K.et al Hashimoto's encephalopathy: a steroid‐responsive disorder associated with high anti‐thyroid antibody titers—report of 5 cases. Neurology 199141228–233. [DOI] [PubMed] [Google Scholar]
- 2.Ferracci F, Bertiato G, Moretto G. Hashimoto's encephalopathy: epidemiologic data and pathogenetic considerations. J Neurol Sci 2004217165–168. [DOI] [PubMed] [Google Scholar]
- 3.Fujii A, Yoneda M, Ito T.et al Autoantibodies against the amino terminal of α‐enolase are a useful diagnostic marker of Hashimoto's encephalopathy. J Neuroimmunol 2005162130–136. [DOI] [PubMed] [Google Scholar]
- 4.Saiz A, Arpa J, Sagasta A.et al Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late‐onset insulin‐dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology 1997491026–1030. [DOI] [PubMed] [Google Scholar]
- 5.Selim M, Drachman D A. Ataxia associated with Hashimoto's disease: progressive non‐familial adult onset cereberllar degeneration with autoimmune thyroiditis. J Neurol Neurosurg Neuropsychiatr 20017181–87. [DOI] [PMC free article] [PubMed] [Google Scholar]