Abstract
Background
Acute‐disseminated encephalomyelitis (ADEM) is a demyelinating disorder of the central nervous system, whose epidemiology, clinical presentations and functional outcome are incompletely understood in Asian populations.
Objective
To assess the clinical presentations, predisposing factors and functional outcome of ADEM in Taiwan.
Methods
50 patients initially diagnosed with ADEM (male, 19; female, 31) were enrolled from 1991 to 2005. Diagnosis of ADEM or multiple sclerosis was established during a follow‐up period of 2–120 months. 8 adult patients were noted to have taken the immunomodulatory drug, levamisole, within 3 months before onset of symptoms. The remaining 42 patients (male, 17; female, 25) were categorised by age as children (<16 years, n = 12), young adults (16–49 years, n = 21) and elderly adults (⩾50 years, n = 9). The clinical manifestations, predisposing factors and radiological findings were compared between different age groups and adult patients with or without levamisole use. Functional outcome was compared by a log‐rank test.
Results
Preceding upper respiratory tract infection was evident in 21 (50%) patients and only one young‐adult patient had received Rubella vaccine immunisation. The frequency of fever was higher in children (p = 0.04) and psychiatric symptoms were more prevalent in elderly patients (p = 0.03). Functional recovery was faster in children than in adults (p = 0.002). Initial Expanded Disability Status Scale score (odds ratio (OR) 1.9, p = 0.03) and no fever (OR 0.04, p = 0.06) were associated with poor outcome (modified Rankin scale ⩾2). After a mean (SD) follow‐up of 31.8 (9.9) months, 4 (9.5%) patients developed multiple sclerosis (3 (25%) children, 1 (4.7%) young adult, p = 0.03). The neurological disability, radiological and cerebrospinal fluid findings did not differ between patients with and without levamisole use. One elderly adult patient previously receiving levamisole developed multiple sclerosis of relapse‐remitting type after a mean follow‐up period of 36.9 months.
Conclusion
The clinical presentations, functional outcome and risk of developing multiple sclerosis differed between different age groups. Functional recovery was faster in children than in adults. Poor functional outcome was related to initial high Expanded Disability Status Scale score and absence of fever.
Acute disseminated encephalomyelitis (ADEM) is a monophasic inflammatory demyelinating disorder of the central nervous system (CNS). Pathogenesis is suspected to be an autoimmune response to myelin, which is triggered by infection or immunisation via molecular mimicry.1,2 Thus, ADEM may be the clinical counterpart to experimental allergic encephalomyelitis (EAE).3 The exact incidence is not known, but it was reported that the incidence of ADEM among persons aged <20 years residing in San Diego County, California was approximately 0.4/105/year.4 More studies have been conducted in paediatric populations than in elderly adults and most reported series were Caucasians.5,6,7,8 Few large series of ADEM have been published in Asian populations.9
We thus assessed the precipitating factors, clinical presentations, cerebrospinal fluid (CSF) and radiological findings, and long‐term outcome in a cohort of ethnic Taiwanese patients with ADEM in different age groups, and attempted to determine the prognostic factors for poor functional outcome.
Patients and methods
From January 1991 to August 2005, 60 patients with initially diagnosed ADEM were admitted to a medical centre in Taipei, and their medical records were reviewed retrospectively. The follow‐up study was based on chart review and telephone interview. Of these 60 patients, 9 with isolated transverse myelitis and 1 with Sjögren's syndrome were excluded. The remaining 50 patients were grouped according to their age at onset (children, <16 years of age; young adults, 16–49 years of age; and elderly adults, ⩾50 years of age).
Diagnosis of ADEM was according to the operational criteria proposed by Schwarz et al,8 including a presumed acute demyelinating process affecting the CNS without a history of unexplained neurological symptoms, and at least one supratentorial or infratentorial demyelinating lesion without evidence of previous destructive white matter lesions shown on magnetic resonance imaging (MRI). Patients were excluded if CNS infection or an other autoimmune process was suspected, or isolated transverse myelitis or optic neuritis was diagnosed.
The clinical manifestations and outcome in each patient were reviewed in detail, including sex, age at onset, predisposing events, neurological symptoms at presentation, the most severe neurological disability, treatment regimen, therapeutic response and functional outcome. The possible predisposing factors were infection, immunisation or even drugs taken within the last month. New drugs prescribed within 4 weeks of symptom onset were recorded, particularly immunomodulatory drugs. Neurological disability was assessed by Kurtzke's Expanded Disability Status Scale (EDSS),10 some scales of which were scored retrospectively according to the functional disability recorded in the chart records. Because measurement of EDSS is dependent on patients cooperating with a neurological examination, this measurement is not suitable for a comatose or uncooperative patient. Thus EDSS was measured only in patients who could cooperate with a simple neurological examination, including patients with clear consciousness and those whose Glasgow Coma Scale (GCS) scores were ⩾13 and who could follow simple orders to evaluate their neurological functional disabilities, such as motor and sensory deficits. In addition, to minimise the limitation of EDSS measurement in patients with acute CNS disease such as ADEM, we also recorded other detailed neurological presentations except motor and sensory dysfunctions on which EDSS is focused, such as GCS, seizures, psychiatric symptoms, bulbar symptoms, sphincter dysfunction and ataxia. The final functional outcome was evaluated by modified Rankin's scale (mRS).11
CSF examination was performed within 2 days after admission in almost all patients. CSF analysis included leucocyte count, cytology and determination of total protein level. Oligoclonal bands of immunoglobulin (Ig)G were evaluated by isoelectric focusing. CSF culture and microbiological investigation for bacterial and viral infection were also undertaken. All enrolled patients underwent head MRI examination before treatment was initiated. Because our study population was collected over a period of 15 years some patients referred to our institution had MRI scans carried out elsewhere. Therefore, the MRI scans analysed in this study were performed with various scanners, using different scanning protocols and, therefore, might have different magnets, slice thicknesses and interslice gaps. Standard T1‐weighted and T2‐weighted images and images after intravenous injection of gadolinium‐diethylenetriamine penta‐acetic acid (DTPA) were recorded for all enrolled patients. Ten patients underwent additional spinal cord MRI scans in the acute stage because of clinical suspicion of additional myelitis. We scored the following items from the T2‐weighted images: total number of lesions; the presence of supratentorial, periventricular, subcortical, corpus callosum, basal ganglion (including internal capsule), brain stem and optic neuritis. The gadolinium‐DTPA‐enhanced T1‐weighted images were analysed for the number of all enhancing lesions. The number of lesions was assessed by visual identification and was confirmed by senior neuroradiologists, who were blinded to the clinical findings. A follow‐up examination during the initial treatment period was performed if the neurological condition of a patient deteriorated after admission. Because early follow‐up studies were not done systemically, only the first diagnostic MRIs were used for analysis.
Three adult patients also underwent brain biopsy because of diagnostic uncertainty. Histological results showed perivascular inflammatory demyelination changes with foamy macrophages. Malignant cells were not seen and viral particles could not be cultured.
Treatments were diverse, with high‐dose intravenous methylprednisolone remaining the most common regimen. The methylprednisolone regimen for children was 30 mg/kg/day for <30 kg of body weight and 1 g/day for >30 kg of body weight; for adults, it was 500–1000 mg daily for 3 days. No further treatment was given for patients with good response to methylprednisolone; otherwise, plasma exchange (five courses with 1500 ml of plasma exchanged with 5% albumin every other day), plasmapheresis or intravenous immunoglobulin (IVIg, 0.4 g/kg intravenous daily for 5 days) was given.12 One adult patient underwent isolated plasma exchange and three children received isolated IVIg.
During follow‐up, any neurological symptom was documented and the Poser criteria were used to diagnose multiple sclerosis.13 Comparisons between groups were made using the one‐way analysis of variance, with retrospective (Bonferroni) comparison for the continuous variables and Mantel–Haenszel χ2 test for the discrete variables. Fisher's exact test was used for values <5. A significant difference was defined as a value of p<0.05. Time zero for the survival analysis was taken as the date of the first cohort‐defining episode, and the time point was the date when the favourable functional outcome occurred (mRS 0 or 1). For event‐free patients, the follow‐up period ended on the date of last clinic visit, and survival curves were estimated by the Kaplan–Meier method. A log‐rank test was used to compare the difference in functional outcome between the three age groups. A logistic regression analysis was used to analyse independent factors predicting poor functional outcome. All statistical analyses were performed using STATA version 8.0 software.
Results
In all, 50 patients were enroled in the study, including 12 children (male, 6; mean (standard deviation (SD)) age, 8.8 (3.7) years), 23 young adults (male, 7; mean (SD) age, 33.1 (11.4) years), and 15 elderly adults (male, 6; mean (SD) age, 62.4 (12.1) years). The mean (SD) follow‐up period was 31.8 (9.9) months (range, 2–120 months). All patients fulfilled the operational diagnostic criteria for ADEM. However, eight adult patients (2 (8.7%) young adults and 6 (40.0%) elderly adults) were noted to have received the immunomodulatory drug (levamisole) within 3 months before onset of symptoms. These eight patients were discussed separately for their possible diagnosis of levamisole‐related multifocal inflammatory leucoencephalopathy.14,15
Table 1 shows the possible precipitating factors in the three age groups of the remaining 42 patients. Preceding upper respiratory tract infection was present in 50% of children, 33.3% of young adults and 44.4% of elderly adults (p = 0.62). Only one young‐adult patient had received immunisation. We found no between‐group differences in the number and percentage of patients receiving other drugs within 4 weeks. The mean (SD) duration from preceding events to clinical symptoms was 6.2 (3.4) days. There were no identifiable predisposing factors in 15 (35.7%) patients.
Table 1 Predisposing factors in 42 patients with acute disseminated encephalomyelitis.
| All, n = 42 | Children, n = 12 | Young adults, n = 21 | Elderly adults, n = 9 | p Values | |
|---|---|---|---|---|---|
| Age at onset, years | 32.8 (4–90) | 8.8 (4–14) | 33.9 (16–49) | 62.3 (50–90) | — |
| Male | 17 (40.5) | 6 (50.0) | 7 (33.3) | 4 (44.4) | 0.62 |
| Predisposing factors | |||||
| Prior upper respiratory tract infection | 21 (50.0) | 6 (50.0) | 11 (52.4) | 4 (44.4) | 0.92 |
| Prior acute gastroenteritis | 3 (7.1) | 1 (8.3) | 1 (4.8) | 1 (11.1) | 0.81 |
| Prior immunisation | 1 (2.4) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 1.00 |
| No identifiable predisposing factor | 15 (35.7) | 2 (16.7) | 7 (33.3) | 6 (66.7) | 0.37 |
| Interval between initial events and symptoms, days | 6.2 (0–30) | 7.5 (1–30) | 6.5 (0–21) | 3.5 (0–7) | 0.55 |
Values are n (%), except for age and interval (mean (range)).
Table 2 shows the initial clinical symptoms in the three age groups. Acute motor weakness was the most common presenting symptom (76.2%), followed by decreased verbal output or mutism (73.8%) and disturbed consciousness (69.0%). Fever was present more frequently in children than in young and elderly adults (75.0% v 33.3% and 33.3%; p = 0.04) and psychiatric symptoms, such as visual hallucination and delusion, were more frequently noted in the elderly patients (none in children and young adults; 33.3% in elderly adults; p = 0.03). Four young adult patients needed ventilator support due to respiratory failure, as against none in the children and elderly adults (p = 0.16). EDSS was measured only in patients who could cooperate with a simple neurological examination, including 7 (58.3%) children, 15 (71.4%) young adults and 7 (77.8%) elderly adults. The mean (SD) EDSS was 6.8 (2.1) in children, 6.4 (2.8) in young adults and 6.2 (2.1) in elderly adults (p = 0.43).
Table 2 Clinical symptoms of 42 patients with acute‐disseminated encephalomyelitis.
| All, n = 42 | Children, n = 12 | Young adults, n = 21 | Elderly adults, n = 9 | p Value | |
|---|---|---|---|---|---|
| Fever | 29 (45.2) | 9 (75.0) | 7 (33.3) | 3 (33.3) | 0.04 |
| Disturbed consciousness | 29 (69.0) | 7 (58.3) | 15 (71.4) | 7 (77.8) | 0.60 |
| GCS, mean (SD) | 12.7 (2.6) | 12.6 (2.7) | 12.4 (3.2) | 13.5 (2.2) | 0.52 |
| Seizure | 12 (28.6) | 6 (50) | 4 (19.0) | 2 (22.2) | 0.15 |
| Psychiatric symptoms | 3 (7.1) | 0 (0) | 0 (0) | 3 (33.3) | 0.03 |
| Decreased visual acuity | 3 (7.1) | 2 (16.7) | 1 (4.8) | 0 (0) | 0.42 |
| Decreased verbal output or mute | 31 (73.8) | 10 (83.3) | 15 (71.4) | 6 (66.7) | 0.67 |
| Motor weakness | 32 (76.2) | 11 (91.7) | 14 (66.7) | 7 (77.8) | 0.27 |
| Sensory deficit | 12 (28.6) | 3 (25.0) | 7 (33.3) | 2 (22.2) | 0.78 |
| Extrapyramidal | 8 (19.1) | 3 (25.0) | 4 (19.1) | 1 (11.1) | 0.78 |
| Bulbar symptoms | 27 (64.3) | 9 (75.0) | 13 (61.9) | 5 (55.6) | 0.62 |
| Ataxia | 15 (35.7) | 4 (33.3) | 7 (33.3) | 4 (44.4) | 0.84 |
| Sphincter | 18 (42.9) | 6 (50.0) | 8 (38.1) | 4 (44.4) | 0.92 |
| Respiratory failure | 4 (9.5) | 0 (0) | 4 (19.0) | 0 (0) | 0.16 |
| EDSS*, mean (SD, %) | 6.5 (2.9, 69.0) | 6.8 (2.1, 58.3) | 6.4 (2.8, 71.4) | 6.2 (2.1, 77.8) | 0.43 |
EDSS, Expanded Disability Scale; GCS, Glasgow Coma Scale.
Bulbar symptoms included dysphagia and dysarthria. Values are n (%).
*EDSS score was not measured in all patients due to lack of patient cooperation and consciousness level. It was measured in 7 children, 15 young adults and 7 elderly adults.
CSF abnormalities, including pleocytosis and increased total protein level, were noted in 66.7% of children, 37.6% of young adults and 51.2% of elderly adults (p = 0.35). We found no obvious between‐group differences in total protein and glucose concentrations and leucocyte counts. Intrathecal oligoclonal bands were not noted in all patients.
Most patients had >6 lesions on T2‐weighted images (children, 66.7%; young adults, 76.2%; and elderly adults, 66.7%; p = 0.67; table 3). Gadolinium‐DTPA‐enhanced lesions were present in 41.7% of children, 42.9% of young adults and 33.3% of elderly adults (p = 0.92). Children had less periventricular involvement (25% v 66.7% in young adults and 88.9% in elderly adults, p = 0.01), but more cerebellum (50% v 14.3% in young adults and none in elderly adults, p = 0.009) and spinal cord (50% v 4.8% in young adults and 11.1% in elderly adults, p = 0.005) involvement than adults. Additional spinal cord involvement was present in six children: in two at cervical segments, in two at both thoracic and lumbar segments, in one at both cervical and thoracic segments and in one from cervical to lumbar segments. Only one young adult had additional cervical and thoracic cord involvement and an elderly adult patient had cervical cord involvement.
Table 3 Initial head magnetic resonance image findings in 42 patients with acute‐disseminated encephalomyelitis.
| All, n = 42 | Children, n = 12 | Young adults, n = 21 | Elderly adults, n = 9 | p Value | |
|---|---|---|---|---|---|
| Number of lesions | |||||
| 1–3 | 6 (14.3) | 1 (8.3) | 3 (14.3) | 2 (22.2) | 0.67 |
| 4–6 | 6 (14.3) | 3 (25.0) | 2 (9.5) | 1 (11.1) | |
| >6 | 30 (71.4) | 8 (66.7) | 16 (76.2) | 6 (66.7) | |
| Gd‐enhanced lesions | 17 (40.5) | 5 (41.7) | 9 (42.9) | 3 (33.3) | 0.92 |
| Location of lesion | |||||
| Cortex | 18 (42.9) | 7 (58.3) | 8 (38.1) | 3 (33.3) | 0.51 |
| Periventricular areas | 25 (59.5) | 3 (25.0) | 14 (66.7) | 8 (88.9) | 0.01 |
| Subcortical areas | 20 (47.6) | 5 (41.7) | 11 (52.4) | 4 (44.5) | 0.92 |
| Corpus callosum | 4 (9.5) | 2 (16.7) | 2 (9.5) | 0 (0) | 0.66 |
| Basal ganglion | 26 (61.9) | 10 (83.3) | 12 (57.1) | 4 (44.4) | 0.19 |
| Optic nerve | 3 (7.1) | 2 (16.7) | 1 (4.8) | 0 (0.0) | 0.42 |
| Midbrain or pons | 23 (54.8) | 8 (66.7) | 12 (57.1) | 3 (33.3) | 0.39 |
| Medulla | 14 (33.3) | 1 (8.3) | 11 (52.4) | 2 (22.2) | 0.03 |
| Cerebellum | 10 (23.8) | 6 (50.0) | 3 (14.3) | 0 (0.0) | 0.009 |
| Spinal cord | 8 (19.0) | 6 (50.0) | 1 (4.8) | 1 (11.1) | 0.005 |
Gd, gadolinium‐diethylenetriaminepenta‐acetic acid.
Values are n (%).
The average interval from onset to treatment was 15.3 (17.2) days. Most patients received intravenous methylprednisolone treatment (children, 67.7%; young adults, 78.1%; and elderly adults, 67.5%). Four young adults and three children who did not respond well to methylprednisolone received plasma exchange/plasmapheresis or IVIg. One elderly patient received plasma exchange and three children received IVIg solely, without preceding methylprednisolone treatment.
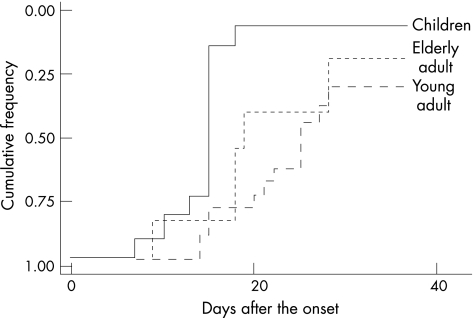
The mean (SD) follow‐up time was 22.5 (2.8) months in children, 28.5 (6.7) months in young adults and 31.3 (7.5) months in elderly adults. The frequency of good functional outcome (mRS <2) was higher in children than in adults (children, 100%; young adults, 66.7%; and elderly adults, 75.0%; p = 0.08). The Kaplan–Meier curves showed that the cumulative frequencies of mRS (0 or 1) were higher in children than in adults (fig 1, p = 0.002 by log‐rank test). Logistic regression analysis showed that initial high EDSS (OR 1.9, p = 0.03) and absence of fever (OR 0.04, p = 0.06) were associated with poor functional outcome (mRS ⩾2 or death). After a mean (SD) follow‐up of 31.8 (9.9) months, 4 (9.5%) patients developed multiple sclerosis, including 3 (25%) children and, 1 (4.7%) young adult but no elderly adult (p = 0.03). Three of the patients had the relapse‐remitting type, and one child developed the secondary progressive form of multiple sclerosis.
Figure 1 Kaplan–Meier curves for final favourable functional status (modified Rankin scale <2) by three age groups (p = 0.002, calculated with the use of the log‐rank test).
Discussion
To our knowledge, this is the first study to compare clinical and radiological findings and functional outcome in different age groups of patients with ADEM in a sizeable Asian population. Our 42 patients fulfilled the operational diagnostic criteria for ADEM, including a single clinical episode and radiological evidence of demyelination of the CNS, although four patients developed multiple sclerosis during follow‐up. Unlike previous reports, our study included patients of all ages with ADEM. The most common predisposing event in the three age groups was preceding upper respiratory tract infection, but only less than one tenth of all patients had symptoms of acute gastroenteritis (vomiting and diarrhoea) without intergroup difference. Compared with previous studies conducted in children and adolescences, the percentages of preceding events are similar in our study, although Leake et al reported a much higher incidence rate of preceding vomiting.4,5
However, previous use of immunomodulatory drugs, particularly levamisole, seemed to have some role in inciting demyelination in elderly adults in our study population. Eight adult patients (two young adults and six elderly adults) had received levamisole within 3 months before onset of neurological symptoms. The mean duration of levamisole use was 47.6 days (range 3–150 days) and the average total dose was 5475 mg (range 450–13 500 mg). Because it was difficult to differentiate levamisole‐related multifocal inflammatory leucoencephalopathy from ADEM in these eight patients, we separated these cases from other adult patients with ADEM and compared the clinical presentation, radiological findings, CSF studies and functional outcome between these two groups, as depicted in table 4. The mean EDSS, MRI and CSF findings were similar in both groups, with evidence of multiple inflammation‐enhancing white matter lesions with a predilection for periventricular areas. The functional outcome was favourable in patients with previous levamisole use. However, it is interesting to note that one elderly adult patient with previous levamisole use developed multiple sclerosis of the relapse‐remitting type after a mean follow‐up period of 36.9 months. Levamisole was originally developed as an anti‐helmintic and is now used as adjuvant chemotherapy with 5‐fluorouracil for colon–rectal cancer.16 It has also been used to treat oral aphthous ulcerations, as in our eight patients.17 It has a broad range of immunomodulatory activity and can augment cellular immune responses, chemotaxis and delayed hypersensitivity.18 Both ADEM and levamisole‐related multifocal inflammatory leucoencephalopathy present with acute to subacute onset of neurological symptoms and multiple white matter lesions shown on MRI. Even brain biopsy cannot differentiate these two entities.15 Our findings also confirmed the diagnostic difficulty of distinguishing between these two disease entities. Further large series enroling more patients with levamisole use are needed to clarify the relationship between levamisole use and CNS demyelination and the development of multiple sclerosis.
Table 4 Clinical characteristics and functional outcome of adult patients with and without levamisole use .
| All adult patients with ADEM, n = 30 | Patients with levamisole use, n = 8 | p Value | |
|---|---|---|---|
| Age at onset (years) | 42.4 (16–90) | 53.1 (24–82) | 0.15 |
| Male | 11 (36.7) | 2 (25.0) | 0.43 |
| EDSS | 6.3–2.6 | 7.1–2.7 | 0.42 |
| Head MRI findings | |||
| Number of lesions | |||
| 1–3 | 5 (16.7) | 0 (0) | 0.40 |
| 4–6 | 3 (10.0) | 1 (12.5) | |
| >6 | 22 (73.3) | 7 (87.5) | |
| Gd‐enhanced lesions | 12 (40.0) | 5 (62.5) | 0.26 |
| CSF findings | |||
| Pleocytosis | 7 (23.3) | 2 (25.0) | 0.66 |
| L predominant | 6 (20.0) | 3 (37.5) | 0.70 |
| Protein (mg/dl; range) | 12 (40.0) | 1 (12.5) | 0.38 |
| Functional outcome | |||
| Rankin scale ⩾2 and death | 9 (30.0) | 1 (12.5) | 0.29 |
| Progress to MS | 1 (3.3) | 1 (12.5) | 0.39 |
| Follow‐up period (months) | 36.7 (20.2) | 36.9 (16.1) | 0.82 |
ADEM, acute‐disseminated encephalomyelitis; CSF, cerebrospinal fluid; DTPA, diethylenetriaminepenta‐acetic acid; EDSS, Expanded Disability Status Scale; Gd, gadolinium; MRI, magnetic resonance imaging; MS, multiple sclerosis.
Values are n (%) except age (mean (range)).
Acute hemiparesis was the most frequent presenting neurological disorder in the three age groups, followed by impaired consciousness in both young and elderly adult groups and decreased verbal output/mutism in children, as in previous studies.4,5,6 Fever was more common in children than in adult patients during the acute period, implying that age‐related changes in immune response, especially inflammatory cytokine reactions, might partially explain this difference. Otherwise, psychiatric symptoms such as visual hallucinations and delusion were more common in the elderly patients than in children and young adults in our study. The more common psychiatric symptoms in the elderly patients can be explained by an emotional reaction to a possibly fatal illness, although symptoms may also be due to damage to the limbic system caused by the disease process.
In our study, there was no significant difference in CSF findings between groups, and no patient (even those who later developed multiple sclerosis) had increased oligoclonal bands. The low incidence of CSF oligoclonal bands in our study, both in children and adults, was different from that in previous studies conducted in Caucasian adult patients where more than half of the patients had increased IgG oligoclonal bands.8 Compared with the low incidence of CSF IgG oligoclonal bands in our patients with ADEM, the frequency of IgG oligoclonal bands in patients with multiple sclerosis across Asia ranged from 30% to 60%, suggesting that CSF oligoclonal bands might discriminate ADEM from multiple sclerosis in Asian populations.19
In this study, we found no between‐age group differences in the number of lesions and extent of their enhancement on MRI but the distribution of demyelinating lesions differed. Children had more frequent involvement of the cerebellum and spinal cord, and less frequent involvement of the periventricular areas. They also had more involvement of basal ganglion and cortical lesions than adult patients (although this, did not reach significance), as in other studies.20 Previous MRI studies of ADEM often focussed on its differentiation from multiple sclerosis.21,22 Lesions in children with ADEM tend to be in the subcortical white matter, whereas lesions in children with multiple sclerosis tend to be both in the subcortical white matter and in the periventricular margin.23,24 But our study did not support the above findings. The extent of periventricular involvement did not differ between children who did and did not develop multiple sclerosis, although the sample size was small and longer follow‐up was needed.
Children had a more favourable functional outcome and faster recovery than adults. Age‐dependent differences in the immune response may result in differences in the development of demyelination. The results from animal studies on EAE showed that middle‐aged C57BL/6 male mice had more severe symptoms and were more refractory to treatment than young mice.25 The more severe EAE observed in middle‐aged mice was likely influenced by changes in the distribution and function of splenic lymphocytes and by a marked reduction in the number of suppressor T cells.25 Otherwise, dysregulation of T cell function in the elderly patients, the causes of which are multifactorial, might partially explain the differences in functional outcome between children and adults.26 In our study, we did not find a significant association between functional disability and age at onset, but initial high EDSS score and absence of fever were associated with poor functional outcome (mRS >2 and death) in all three groups, findings which are consistent with a previous report on patients conducted in a paediatric population.7 Higher initial EDSS score may imply a more aggressive course of immune dysfunction and more neurological sequelae. Because patients who could not cooperate with clinical examinations and had a poor consciousness level did not undergo EDSS measurement, we also correlated the GCS score with functional outcome but did not find any significant association. Fever is associated with protective inflammatory cytokines triggered by multiple immune responses against foreign antigens, and is also a reason for seeking medical advice. Diagnosis is delayed by the absence of fever, which may partly explain our finding.
Of our 42 patients, 4 (9.5%) developed multiple sclerosis over a mean follow‐up of 31.8 months, including 3 (25%) children and 1 (4.7%) young adult. These frequencies, both in children and adults, were much lower than those in Caucasian patients. A previous study in Germany showed that 14 (35%) of 40 adults with ADEM developed multiple sclerosis over a mean period of 38 months.8 A study in France reported that 57% of children with ADEM developed multiple sclerosis after a follow‐up of 2.9 years.5
In conclusion, clinical presentations, functional outcome and risk of development of multiple sclerosis differed between different age groups with ADEM in the Asian population. Functional recovery was faster in children than in adults. Poor functional outcome was related to initial high EDSS score and absence of fever.
Abbreviations
ADEM - acute disseminated encephalomyelitis
CNS - central nervous system
CSF - cerebrospinal fluid
DTPA - diethylenetriamine penta‐acetic acid
EAE - experimental allergic encephalomyelitis
EDSS - Expanded Disability Status Scale
GCS - Glasgow Coma Scale
IVIg - intravenous immunoglobulin
MRI - magnetic resonance imaging
mRS - modified Rankin's scale
Footnotes
Competing interests: None.
References
- 1.Jarousse N, Viktorova E G, Pilipenko E V.et al An attenuated variant of the GDVII strain of Theiler's virus does not persist and infect the white matter of the central nervous system. J Virol 199973801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolley N D, Tsunoda I, Fujinami R S. DNA vaccination against Theiler's murine encephalomyelitis virus leads to alterations in demyelinating disease. J Virol 199973993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorens P G, VanderBorght A, Ceulemans B.et al Encephalomyelitis‐associated antimyelin autoreactivity induced by streptococcal exotoxins. Neurology 2000541433–1441. [DOI] [PubMed] [Google Scholar]
- 4.Leake A D, Albani S, Kao A S.et al Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis 200423756–764. [DOI] [PubMed] [Google Scholar]
- 5.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long‐term follow‐up study of 84 pediatric patients. Neurology 2002591224–1231. [DOI] [PubMed] [Google Scholar]
- 6.Dale R C, de Sousa C, Chong W K.et al Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain 20001232407–2422. [DOI] [PubMed] [Google Scholar]
- 7.Mikaeloff Y, Suissa S, Valleé L.et al First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr 2004144246–252. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz S, Mohr A, Knauth M.et al Acute disseminated encephalomyelitis: a follow‐up study of 40 adult patients. Neurology 2001561313–1318. [DOI] [PubMed] [Google Scholar]
- 9.Murthy J M, Yangala R, Meena A K.et al Clinical, electrophysiological and magnetic imaging study of acute disseminated encephalomyelitis. J Assoc Physicians India 199947280–283. [PubMed] [Google Scholar]
- 10.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 11.Rankin J. Cerebral vascular accidents in patients over the age of 60: prognosis. Scott Med J 19572200–215. [DOI] [PubMed] [Google Scholar]
- 12.Lin C H, Jeng J S, Yip P K. Plasmapheresis in acute disseminated encephalomyelitis. J Clin Apheresis 200419154–159. [DOI] [PubMed] [Google Scholar]
- 13.Poser C M, Paty D W, Scheinberg L.et al New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 198313227–231. [DOI] [PubMed] [Google Scholar]
- 14.Hook C C, Kimmel D W, Kvols L K.et al Multifocal inflammatory leukoencephalopathy with 5‐fluorouracil and levamisole. Ann Neurol 199231262–267. [DOI] [PubMed] [Google Scholar]
- 15.Savarese D M, Gordon J, Smith T W.et al Cerebral demyelination syndrome in a patient treated with 5‐fluorouracil and levamisole. Cancer 199677387–394. [DOI] [PubMed] [Google Scholar]
- 16.Moertel C G, Fleming T R, MacDonal J S.et al Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990322352–359. [DOI] [PubMed] [Google Scholar]
- 17.Sun A, Chia J S, Chang Y F.et al Levamisole and Chinese medical herbs can modulate the serum interleukin‐6 level in patients with recurrent aphthous ulcerations. J Oral Pathol Med 200332206–214. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson H C, Green I, Hamilton J M.et al Levamisole: known effects on the immune system, clinical results, and future applications to the treatment of cancer. J Clin Oncol 199992052–2066. [DOI] [PubMed] [Google Scholar]
- 19.Kira J I. Multiple sclerosis in the Japanese population. Lancet Neurol 20032117–127. [DOI] [PubMed] [Google Scholar]
- 20.Hynson J L, Kornberg A J, Coleman L T.et al Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology 2001561308–1312. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S, Nezu A, Ohtsuki N.et al Serial magnetic resonance imaging in children with postinfectious encephalitis. Brain Dev 199618461–465. [DOI] [PubMed] [Google Scholar]
- 22.Mader I, Stock K W, Ettlin T.et al Acute disseminated encephalomyelitis: MR and CT features. Am J Neuroradiol 199617104–109. [PMC free article] [PubMed] [Google Scholar]
- 23.Boutin B, Esquivel E, Mayer M.et al Multiple sclerosis in children: report of clinical and paraclinical features of 19 cases. Neuropediatrics 198819118–123. [DOI] [PubMed] [Google Scholar]
- 24.Sindern E, Haas J, Stark E.et al Early onset MS under the age of 16: clinical and paraclinical features. Acta Neurol Scand 199286280–284. [DOI] [PubMed] [Google Scholar]
- 25.Matejuk A, Hopke C, Vandenbark A A.et al Middle‐age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol 20051742387–2395. [DOI] [PubMed] [Google Scholar]
- 26.Fulop T, Larbi A, Wikby A.et al Dysregulation of T‐cell function in the elderly: scientific basis and clinical implications. Drugs Aging 200522589–603. [DOI] [PubMed] [Google Scholar]