Abstract
Objectives
To elucidate the importance of clinically diagnosed cerebral comorbidity in idiopathic normal‐pressure hydrocephalus (INPH) and its effect on improvement after shunt surgery as well as concordance with parenchymal pathological changes described in frontal cerebral biopsy specimens.
Methods
In 28 consecutive patients diagnosed with INPH and shunted according to clinical, radiological and cerebrospinal fluid dynamic criteria, concomitant disorders were carefully registered, with special emphasis on cerebrovascular disease (CVD) and possible Alzheimer's disease. During shunt surgery, a frontal cerebral biopsy specimen was obtained and subsequently analysed for pathological changes.
Results
One or several concurrent disorders were present in 89% of the patients, most often CVD (n = 17) and possible Alzheimer's disease (n = 12), of which eight patients presented both, diagnosed according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association. The shunt success rate was 33%. A clear tendency towards increasing prevalence of CVD or Alzheimer's disease was found in the subgroups with no improvement or clinical deterioration compared with the patients improving after shunt surgery. The presence of CVD tended towards an unfavourable shunt outcome. The pathological parenchymal changes reflected the clinical diagnoses of comorbidity, and were described in about half of the biopsy specimens, with Alzheimer's disease (n = 7) and vascular changes (n = 7) being the most common findings. However, no significant correlation was found with the clinical diagnoses of Alzheimer's disease and CVD. The presence of cerebral comorbidity, whether diagnosed clinically or by brain biopsy, did not preclude clinical improvement after shunt operation.
Conclusions
A high prevalence of CVD and Alzheimer's disease was found in patients shunted for INPH, which was reflected, although less commonly, by similar neuropathological biopsy findings. No significant correlation was found between the presence of comorbidity and shunt outcome. The findings support the perception of INPH as a multiaetiological clinical entity, possibly overlapping pathophysiologically with CVD and Alzheimer's disease.
Patients with the idiopathic normal‐pressure hydrocephalus (INPH) syndrome are traditionally described as presenting a clinical triad of progressing gait disturbances, dementia and urinary urge incontinence, and with a radiologically defined hydrocephalus on computed tomography or magnetic resonance imaging.
As opposed to secondary normal‐pressure hydrocephalus (NPH), no apparent precipitating factor is identified in INPH. The patients are generally older1 and consequently more prone to have concomitant diseases. In concordance, the shunt success rate in patients with possible INPH is lower than in patients with secondary NPH,2,3 varying from 25% to 80%.4,5
In patients not responding to shunt surgery, cerebrovascular disease (CVD) and Alzheimer's Disease are the predominant disorders thought to mimic the INPH syndrome. This presumption is based on the fact that Alzheimer's disease and CVD are the most common causes of dementia, and from autopsy and biopsy findings of CVD or Alzheimer's disease in patients with INPH—including patients improving after a shunt operation.6,7,8,9,10,11 It has also been hypothesised that INPH shares common pathophysiology with Alzheimer's disease12 or CVD,13,14,15 which only further confounds the clinical picture.
Thus, the main question for the clinician contemplating a shunt operation is not whether the patient has INPH or a concurrent irreversible disorder, but whether the hydrocephalus or the presence of a non‐shunt responsive comorbidity—most often CVD or Alzheimer's disease—is the major contributor to the symptomatology. The selection of patients for shunt surgery has become even more controversial after recent data suggesting shunt improvement in patients with no symptoms or signs of INPH but with a clinical diagnosis of Alzheimer's disease.16
In this prospective study of patients shunted for INPH according to clinical, radiological and cerebrospinal fluid (CSF) dynamic criteria, frontal cerebral biopsy specimens were obtained before the insertion of a shunt and examined for pathological parenchymal changes. Clinical comorbidity was carefully registered, and the clinical diagnoses of CVD and Alzheimer's disease were established by a trained neurologist. The presence of concomitant disorders was correlated with shunt outcome and cerebral biopsy findings to elucidate the importance of clinical comorbidity in patients with INPH, and its effect on improvement after shunt surgery as well as concordance with the pathological cerebral parenchymal changes.
Patients and methods
We included 28 consecutive patients (18 men and 10 women; age range 47–77 years, mean 64 years) diagnosed with INPH. The inclusion period was from 1990 to 1992. Exclusion criteria were intracranial haemorrhage or tumour, major cerebral infarction, or a history of subarachnoid bleeding, meningitis, head trauma or intracranial operation.
Informed consent to perform a brain biopsy was obtained from all patients and their relatives. The study was approved by the Danish Central Ethical Committee (protocol number V.100.1500/90).
Study programme
The evaluation programme consisted of a clinical, neurological and neuropsychological examination and scoring by the Mini‐Mental Status Examination (MMSE),17 the Hachinski Ischaemic Score18 and the Global Deterioration Scale (GDS).19 Gait disturbances and urinary incontinence were scored semiquantitatively using ordinal scales with five degrees: gait: 1, normal; 2, abnormal, but possible without support; 3, need of cane; 4, need of support from another person; and 5, wheelchair required; urinary incontinence: 1, none; 2, tendency of urge incontinence; 3, rare episodes of urge incontinence; 4, occasionally; and 5, constant.
Most patients underwent an extensive neuropsychological assessment consisting of a range of tests covering the cognitive domains of memory, concentration, abstract thinking, abstract problem solving, language, visual perception and visual construction. Presence of dementia was determined according to the Diagnostic and statistical manual of mental disorders criteria.20 Intraventricular pressure (ICP) of all patients was monitored for 16–24 h using a catheter in the right frontal horn. Using the same catheter, a subsequent CSF lumboventricular perfusion test21 or a computerised steady rate infusion test was performed.22,23 ICP, B‐wave activity as a percentage of the monitored period, and resistance to CSF outflow (Rout) were used to characterise the CSF dynamics.
Clinical diagnoses
Criteria for the diagnosis of INPH and hence shunt operation were as follows:
Presence of ⩾2 symptoms from the clinical triad
Ventricular enlargement on a cerebral computed tomography scan
Increased Rout (⩾10 mm Hg/ml/min) with or without a B‐wave activity ⩾50% of the monitoring period.24,25
The diagnosis of INPH and hence the decision on shunt surgery were independent of the possible diagnosis of other concomitant conditions or the cerebral biopsy findings.
The presence of concurrent conditions, which potentially could contribute to the symptoms, was evaluated by a neurologist, on the basis of the anamnestic, clinical, laboratory, neuropsychological and radiological findings. The neurologist was unaware of the biopsy findings and shunt outcome. Each patient was diagnosed with “possible”, “uncertain” or “unlikely” Alzheimer's disease according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS–ADRDA) criteria.26 By definition, none of the patients fulfilled the criteria for probable Alzheimer's disease, as primarily they were diagnosed with and treated for INPH.
The diagnosis of CVD was based on a clinical history of stroke, transient ischaemic attacks or radiologically (computed tomography scanning) evident single infarcts, lacunar infarcts or extensive leucoencephalopathy. All other disorders diagnosed during the evaluation programme with a possible contribution to the symptoms were also noted.
Sampling of biopsy specimens
The biopsy specimens (0.5–1.0 cm3) were taken from the right superior frontal cortex and underlying white matter 1–4 weeks after the ICP monitoring and Rout measurement. The same burr hole was used, but tissue affected by the earlier ICP monitoring was avoided and no electrocoagulation was performed before excision. A ventriculoperitoneal shunt insertion was performed during the same surgical procedure.
The biopsy samples were prepared and analysed as described previously.27 A single neuropathologist, blinded to the presence of clinically diagnosed comorbidity or shunt outcome, reviewed the biopsy specimens and assessed the presence of pathological changes in the cerebral parenchyma including the extent of arteriosclerotic vasculopathy referred to as “vascular changes”. Alzheimer's disease changes were said to be present when >10 neuritic plaques/mm2 were observed in the most affected areas of the biopsy sample.28
Follow‐up programme
Patients included in the study were re‐evaluated with neurological examination and scoring of gait, urinary incontinence, GDS and MMSE 3–9 months after the biopsy sampling. A score of +1 was assigned for each degree of improvement and a score of −1 was assigned for each degree of reduction in the ordinal scales of the following variables: gait, incontinence and MMSE score (a change of 5 points in the MMSE score corresponded to a change of 1°). A patient was classified as improved only when a summation of the improvement and reduction scores was ⩾+2. An increase of at least two degrees in the ordinal scales was thus required to classify the patient as improved. The doctor rating the patients was unaware of the biopsy findings. All patients who underwent a shunt operation also underwent a cranial computed tomography scan.
Results
Clinical presentation and biopsy findings
Gait disturbances were present in 86% (n = 24) of the 28 patients. The remaining patients (n = 4) presented both urinary incontinence and cognitive deficits. Urinary incontinence was present in 18 (64%) of all patients. Dementia was present in 21, absent in 4 and uncertain in 3 patients, according to the Diagnostic and statistical manual of mental disorders—third edition revised criteria. Including the three patients presenting with cognitive deficits but not with certainty fulfilling the criteria for dementia, the full clinical triad of gait disturbances, urinary incontinence and cognitive deficits was present in 64% (n = 18) of the patients. The median (range) values of GDS, MMSE and Hachinski Ischaemic Scores were 5 (2–7), 23 (0–30) and 4 (1–12, not performed in one patient), respectively.
Pathological cerebral parenchymal changes were described in 15 (54%) of the 28 biopsy specimens, most often Alzheimer's disease changes (n = 7) or vascular changes (n = 7), which were also present concomitantly in one patient. The remaining pathological findings included non‐specific cortical degeneration (n = 1) and sequelae of cerebral haemorrhage (n = 1). The patients were evenly distributed when subgrouped according to the presence or absence of pathological parenchymal changes and the presence of gait disturbances, urinary incontinence, dementia, full clinical triad, MMSE or GDS scores (table 1).
Table 1 Clinical presentation of 28 patients who underwent a shunt operation for idiopathic normal‐pressure hydrocephalus.
| Cerebral pathology | ||
|---|---|---|
| Absent (n = 13) | Present (n = 15) | |
| Gait disturbances | ||
| Absent (n = 4) | 3 | 1 |
| Present (n = 24) | 10 | 14 |
| Incontinence | ||
| Absent (n = 10) | 5 | 5 |
| Present (n = 18) | 8 | 10 |
| Dementia | ||
| Absent (n = 4) | 2 | 2 |
| Present (n = 24)* | 11 | 13 |
| Full clinical triad | ||
| Absent (n = 10) | 5 | 5 |
| Present (n = 18) | 8 | 10 |
| MMSE median score (range) | 21 (8–30) | 25 (0–30) |
| GDS median score (range) | 6 (4–6) | 4 (2–7) |
| Alzheimer's pathology | ||
|---|---|---|
| Absent (n = 21) | Present (n = 7) | |
| Dementia | ||
| Absent (n = 4) | 4 | 0 |
| Present (n = 24)* | 17 | 7 |
| Full clinical triad | ||
| Absent (n = 10) | 9 | 1 |
| Present (n = 18) | 12 | 6 |
| Unlikely AD (n = 16)† | 12 | 4 |
| Possible AD (n = 12)† | 9 | 3 |
| Vascular changes | ||
|---|---|---|
| Absent (n = 21) | Present (n = 7) | |
| Dementia | ||
| Absent (n = 4) | 2 | 2 |
| Present (n = 24)* | 19 | 5 |
| Full clinical triad | ||
| Absent (n = 10) | 7 | 3 |
| Present (n = 18) | 14 | 4 |
| CVD | ||
| Absent (n = 11)‡ | 9 | 2 |
| Present (n = 17)‡ | 12 | 5 |
| Hachinski median score (range) | 5 (1–7) | 3 (3–5) |
AD, Alzheimer's disease; CVD, cerebrovascular disease; GDS, Global Deterioration Score; MMSE, Mini‐Mental State Examination; NINDCS–ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.
Subgroups according to the presence or absence of pathological findings on the cerebral biopsy and findings specific to Alzheimer's disease or CVD.
* Including three patients presenting with cognitive deficits but not fulfilling criteria for the diagnosis of dementia.
†According to the NICNS–ADRDA criteria.
‡According to clinical and/or radiological findings (described in the text).
Clinical comorbidity
Various concomitant disorders were noted in 89% (n = 25) of the patients, and in most patients they represented a possible contribution to some or all of the symptomatology. The most common clinical comorbidity, CVD, was diagnosed in 61% (n = 17) of the patients, followed by previous alcohol consumption (n = 4), arterial hypertension (n = 4), epilepsy (n = 3), myxoedema (n = 3), vitamin B12 deficiency (n = 2) and other disorders (n = 4).
Applying the NINCDS–ADRDA criteria, 43% (n = 12) of the patients had possible Alzheimer's disease, whereas the remaining 57% (n = 16) presented with unlikely Alzheimer's disease. Concomitant CVD and Alzheimer's disease was diagnosed in 29% (n = 8) of the patients.
Clinical comorbidity correlated with shunt outcome
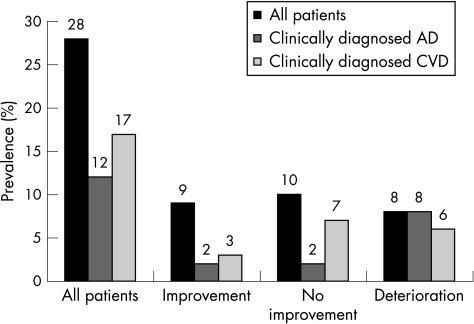
One patient died from pneumonia 1 month after the shunt operation. Of the remaining 27 patients, postoperative clinical improvement was seen in 9 (33%) no change in 10 (37%) and clinical deterioration in 8 (30%). A clear tendency towards increasing frequencies of a clinical diagnosis of CVD or possible Alzheimer's disease can be seen when comparing the subgroup of patients showing improvement with the subgroups of those with no change or those with clinical worsening (fig 1). Only two of the nine improving patients had possible Alzheimer's disease, whereas all eight deteriorating patients also had Alzheimer's disease.
Figure 1 Prevalence among 28 patients diagnosed with idiopathic normal‐pressure hydrocephalus of concomitantly fulfilled clinical criteria for cerebrovascular disease (CVD) or Alzheimer's disease (AD) according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria. Eight patients fulfilled criteria for both CVD and AD. One patient was lost to follow‐up. The patients are subgrouped according to improvement after shunt operation (n = 9), no clinical change (n = 10) and clinical deterioration (n = 8).
When the shunt outcome was dichotomised into improvement/no improvement or deterioration to increase the numbers for statistical analysis (table 2), the clinicoradiological diagnosis of CVD tended to associate with unfavourable shunt outcome (p = 0.053, χ2 test). We found no association between the clinical diagnosis of possible Alzheimer's disease and shunt outcome (table 2).
Table 2 Clinical diagnoses of Alzheimer's disease and cerebrovascular disease correlated with shunt outcome in 27 patients with idiopathic normal‐pressure hydrocephalus.
| No improvement (n = 18) | Improvement (n = 9) | p Value (χ2 test) | |
|---|---|---|---|
| Alzheimer's disease* Unlikely (n = 15) | 8 | 7 | 0.10 |
| Possible (n = 12)* | 10 | 2 | |
| Cerebrovascular disease | |||
| Absent (n = 11) | 5 | 6 | 0.053 |
| Present (n = 16) | 13 | 3 |
*Clinical diagnosis of Alzheimer's disease according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria.
Comparing the improvement rate in the subgroup of 24 patients presenting either clinical CVD or Alzheimer's disease (38%, n = 9/24) with the subgroup of 7 patients not presenting with either clinical Alzheimer's disease or CVD (57%, n = 4/7) likewise showed no significant difference.
Clinical comorbidity correlated with cerebral biopsy findings
We found no association when correlating the presence of dementia, a full clinical triad or the clinical diagnosis of Alzheimer's disease according to the NINCDS–ADRDA criteria with the presence (n = 7) or absence (n = 21) of Alzheimer's disease changes in the cortical biopsy specimens (table 1). Likewise, the clinicoradiological diagnosis of CVD, the Hachinski Ischaemic Score, the presence of dementia and a full clinical triad were not correlated in the subgroups of patients with (n = 7) or without (n = 21) vascular changes in the biopsy specimens (table 1).
Discussion
In patients with a clinicoradiological suspicion of INPH, no distinct supplementary test can yet with certainty predict who will benefit from the shunt operation, although prolonged lumbar CSF drainage has a high sensitivity.29,30 In secondary NPH, it is generally assumed that the underlying cerebral event generates a mechanical obstruction to the CSF absorption at the level of the arachnoid villuses or the cortical subarachnoid space.21 However, in INPH it is debated whether degenerative and arteriosclerosis‐induced parenchymal changes are also involved in the pathophysiology.31,32 Consequently, patients with INPH may present a highly variable clinical picture due to the possible coexistence of various underlying disorders.
In this prospective study of 28 patients who underwent a shunt operation for INPH, we found a high prevalence of clinically diagnosed concomitant disorders, mostly Alzheimer's disease (n = 12), according to the NINCDS–ADRDA criteria, or CVD (n = 17), of whom eight patients presented with both. We found a clear tendency towards increasing prevalence of CVD or been shown to Alzheimer's disease in the subgroups of patients with no improvement or clinical deterioration compared with patients improving after shunt surgery. Although no significant association could be seen, a diagnosis of CVD based on clinical or radiological criteria strongly tended towards an unfavourable shunt outcome. Our findings showed, in accordance with previous studies on patients diagnosed with INPH, an increased prevalence of CVD and established CVD as a predictor of poor outcome.14,15 Likewise, a marked coexistence of Alzheimer's disease has been reported in patients with INPH, although on the basis of autopsy and cerebral biopsy findings rather than clinical diagnoses.9,11,33
In our patients, the high prevalence of CVD and Alzheimer's disease based on clinical criteria was also reflected by the cerebral biopsy findings. Pathological parenchymal changes were described in more than half of the 28 biopsy specimens, with Alzheimer's changes (n = 7) and vascular changes (n = 7) being the most common. However, we could not show any major correlation when comparing the presence or absence of vascular‐related changes in the biopsy specimens with the Hachinski Ischaemic Score or the clinicoradiological diagnosis of CVD, or when comparing the presence or absence of Alzheimer's changes with the clinical diagnosis of Alzheimer's disease according to the NINCDS–ADRDA criteria. A comparison of our biopsy findings with the results of CSF dynamics and shunt outcome has been published previously.7,27
The apparently low clinicopathological correlation of CVD and Alzheimer's disease may be explained by several factors. Firstly, the present patient cohort was highly selective. All patients were referred for and diagnosed with INPH, the symptoms of which therefore may have confounded the clinical picture. Secondly, the generally higher correlation of brain pathology with the NINCDS–ADRDA criteria34 is normally based on consecutive autopsy slices rather than a single frontal biopsy sample. Thirdly, although the neuritic plaques of Alzheimer's disease are rarely seen in the frontal cortex in normal autopsy cases, a single frontal biopsy without Alzheimer's disease changes cannot with certainty exclude the possibility of the disease. Conversely, the findings of Alzheimer's disease changes in a frontal biopsy specimen allows for a definite diagnosis of Alzheimer's disease, as they are virtually invariably seen in the frontal lobes in patients with Alzheimer's disease.35,36 However, these changes and the vascular changes described in the present cerebral biopsy specimens may not yet have been sufficiently extensive to cause clinical symptoms resulting in the fulfilment of the clinical criteria for the diagnoses.
The shunt success rate in our study was 33%, which we consider rather low, although consistent with shunt results from other studies.4,37 The low improvement rate may be due to the applied CSF dynamic shunt criteria, as all patients with a resistance to the cerebral outflow, Rout, above 10 mm Hg/ml/min underwent a shunt operation. However, normal Rout values have subsequently been shown to increase with age,38,39 and a higher cut‐off value is currently recommended.40,41 Accordingly, we have applied an Rout cut‐off value ⩾16 mm Hg/ml/min in our later studies, when comorbidity was present, and higher improvement rates of 72% and 76% were obtained.42,43 Furthermore, we used rather strict outcome measures including functional scales to classify the postoperative clinical outcome. Using functional scales tends to result in a less positive rating of shunt outcome.37
Several of our patients also improved cognitively, despite a clinical or biopsy‐diagnosed comorbidity of CVD or Alzheimer's disease. We therefore underscore that diagnosing CVD and Alzheimer's disease during the preoperative evaluation of patients suspected to have INPH does not necessarily contradict improvement by CSF shunting. Even performing a brain biopsy with analysis of degenerative or vascular parenchymal changes does not add any decisively predictive information on the shunt effect, although the opposite has been suggested.33
Our findings of a high prevalence of CVD and possible Alzheimer's disease diagnosed both clinically and by cerebral biopsies, along with a lack of correlation with shunt outcome, are compatible with the hypothesis of INPH as a multifactorial syndrome. The prevalence of biopsy‐proved Alzheimer's disease in our patients with INPH with a mean age of 64 years was 25%. This number is much higher than expected, if the disorders were unrelated, as the prevalence of Alzheimer's disease is about 10% in a general population aged ⩾65 years. The underlying pathophysiology of INPH thus seems to involve a wide spectrum, ranging from purely hydrodynamic disturbances to major cerebral parenchymal changes, with no manifest association between the clinical presentation, the cerebral parenchymal findings and shunt outcome. Consequently, INPH may share pathophysiological elements with both Alzheimer's disease and CVD. In CVD, more specifically subcortical arteriosclerotic encephalopathy (SAE), it could be increased white‐matter microangiopathy.44 In Alzheimer's disease, it could be CSF stasis resulting in reduced clearance of potentially neurotoxic macromolecules.32 Supporting this view, studies have also indicated that vascular dementia and Alzheimer's disease share common risk factors, possibly also reflecting common pathogenic pathways.45,46
INPH, SAE and Alzheimer's disease may thus pathophysiologically represent overlapping conditions, raising the possibility of common treatment potentials. The interesting question of whether shunting in patients with Alzheimer's disease or SAE may stabilise an otherwise progressive cognitive decline or even improve the cognitive deficits has already been raised.16,44 Obviously, this only adds to the complexity of selecting patients with possible INPH for shunt surgery.
In conclusion, we found the following:
The clinical presentation of patients who undergo a shunt operation for INPH is characterised by a high prevalence of comorbidity, as about half of the patients fulfilled clinical criteria for CVD and Alzheimer's disease.
The clinically diagnosed comorbidity was reflected, although less commonly, by similar neuropathological findings of Alzheimer's disease and vascular changes, when the diagnostic investigation was supplemented with a brain biopsy.
Even with special emphasis on the presence of concomitant clinical conditions and the presence of the cerebral pathological changes, we did not encounter a possible consistent characterisation of the shunt‐responsive patients with INPH. However, clinically diagnosed CVD tended to predict a poor outcome after shunt surgery. The findings support the perception of INPH as a multiaetiological clinical entity, possibly overlapping pathophysiologically with CVD and Alzheimer's disease.
Acknowledgements
We thank neuropathologist Dr Leif Klinken for his contribution to this study.
Abbreviations
CSF - cerebrospinal fluid
CVD - cerebrovascular disease
GDS - Global Deterioration Score
ICP - intracranial pressure
INPH - idiopathic normal‐pressure hydrocephalus
MMSE - Mini‐Mental Scale Examination
NINCDS–ADRDA - National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association
NPH - normal‐pressure hydrocephalus
SAE - subcortical arteriosclerotic encephalopathy
Footnotes
Funding: This study was funded by a grant from the Foundation of Grocer Knud and wife Marie Øster‐Jørgensen for research on hydrocephalus.
Competing interests: None.
References
- 1.Greenberg J O, Shenkin H A, Adam R. Idiopathic normal pressure hydrocephalus—a report of 73 patients. J Neurol Neurosurg Psychiatry 197740336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomsen A M, Børgesen S E, Bruhn P.et al Prognosis of dementia in normal‐pressure hydrocephalus after a shunt operation. Ann Neurol 198620304–310. [DOI] [PubMed] [Google Scholar]
- 3.Larsson A, Wikkelsø C, Bilting M.et al Clinical parameters in 74 consecutive patients shunt operated for normal pressure hydrocephalus. Acta Neurol Scand 199184475–482. [DOI] [PubMed] [Google Scholar]
- 4.Vanneste J, Augustijn P, Dirven C.et al Shunting normal pressure hydrocephalus: do the benefits outweigh the risks? Neurology 19924254–59. [DOI] [PubMed] [Google Scholar]
- 5.Hebb A O, Cusimano M D. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 2001491166–1184. [DOI] [PubMed] [Google Scholar]
- 6.Akai K, Uchigasaki S, Tanaka U.et al Normal pressure hydrocephalus neuropathological study. Acta Pathol Jpn 19873797–110. [PubMed] [Google Scholar]
- 7.Bech R A, Waldemar G, Gjerris F.et al Shunting effects in patients with idiopathic normal pressure hydrocephalus; correlation with cerebral and leptomeningeal biopsy findings. Acta Neurochir (Wien) 1999141633–639. [DOI] [PubMed] [Google Scholar]
- 8.Brusa G, Piccardo A, Pizio N.et al Anatomopathological study of dementia syndrome linked with an abnormal cerebrospinal fluid flow. Pathologica 199183351–358. [PubMed] [Google Scholar]
- 9.Del Bigio M R, Cardoso E R, Halliday W C. Neuropathological changes in chronic adult hydrocephalus: cortical biopsies and autopsy findings. Can J Neurol Sci 199724121–126. [DOI] [PubMed] [Google Scholar]
- 10.Di Rocco C, Di Trapani G, Maira G.et al Anatomo‐clinical correlations in normotensive hydrocephalus: report on three cases. J Neurol Sci 197733437–452. [DOI] [PubMed] [Google Scholar]
- 11.Golomb J, Wisoff J, Miller D C.et al Alzheimer's disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry 200068778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverberg G D, Mayo M, Saul T.et al Alzheimer's disease, normal‐pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol 20032506–511. [DOI] [PubMed] [Google Scholar]
- 13.Koto A, Rosenberg G, Zingesser L H.et al Syndrome of normal pressure hydrocephalus: possible relation to hypertensive and arteriosclerotic vasculopathy. J Neurol Neurosurg Psychiatry 19774073–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauss J K, Regel J P, Vach W.et al Vascular risk factors and arteriosclerotic disease in idiopathic normal‐pressure hydrocephalus of the elderly. Stroke 19962724–29. [DOI] [PubMed] [Google Scholar]
- 15.Boon A J, Tans J T, Delwel E J.et al Dutch Normal‐Pressure Hydrocephalus Study: the role of cerebrovascular disease. J Neurosurg 199990221–226. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg G D, Levinthal E, Sullivan E V.et al Assessment of low‐flow CSF drainage as a treatment for AD: results of a randomized pilot study. Neurology 2002591139–1145. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M F, Folstein S E, McHugh P R. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 18.Hachinski V C, Iliff L D, Zilhka E.et al Cerebral blood flow in dementia. Arch Neurol 197532632–637. [DOI] [PubMed] [Google Scholar]
- 19.Reisberg B, Ferris S H, de Leon M.et al The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry 19821391136–1139. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association Diagnostic and statistical manual of mental disorders, DSM III. Washington, DC: APA, 1987
- 21.Gjerris F, Børgesen S E. Pathophysiology of the CSF circulation. In: Crockard A, Hayward RD, Hoff JT, eds. Neurosurgery—the scientific basis of clinical practice. Boston: Blackwell Science, 2000147–168.
- 22.Børgesen S E, Albeck M J, Gjerris F.et al Computerized infusion test compared to steady pressure constant infusion test in measurement of resistance to CSF outflow. Acta Neurochir Wien 199211912–16. [DOI] [PubMed] [Google Scholar]
- 23.Czosnyka M, Whitehouse H, Smielewski P.et al Testing of cerebrospinal compensatory reserve in shunted and non‐ shunted patients: a guide to interpretation based on an observational study. J Neurol Neurosurg Psychiatry 199660549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Børgesen S E, Gjerris F. The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain 198210565–86. [DOI] [PubMed] [Google Scholar]
- 25.Lundar T, Nornes H. Determination of ventricular fluid outflow resistance in patients with ventriculomegaly. J Neurol Neurosurg Psychiatry 199053896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M F.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 27.Bech R A, Juhler M, Waldemar G.et al Frontal brain and leptomeningeal biopsy specimens correlated with cerebrospinal fluid outflow resistance and B‐wave activity in patients suspected of normal‐pressure hydrocephalus. Neurosurgery 199740497–502. [DOI] [PubMed] [Google Scholar]
- 28.Khachaturian Z S. Diagnosis of Alzheimer's disease. Arch Neurol 1985421097–1105. [DOI] [PubMed] [Google Scholar]
- 29.Marmarou A, Young H F, Aygok G A.et al Diagnosis and management of idiopathic normal‐pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg 2005102987–997. [DOI] [PubMed] [Google Scholar]
- 30.Mori K. Management of idiopathic normal‐pressure hydrocephalus: a multiinstitutional study conducted in Japan. J Neurosurg 200195970–973. [DOI] [PubMed] [Google Scholar]
- 31.Bradley W G, Whittemore A R, Whatanabe A S.et al Association of deep white matter infarction with chronic communicating hydrocephalus: implications regarding the possible etiology of normal pressure hydrocephalus. Am J Neuro Radiol 19911231–39. [PMC free article] [PubMed] [Google Scholar]
- 32.Silverberg G D. Normal pressure hydrocephalus (NPH): ischaemia, CSF stagnation or both. Brain 2004127947–948. [DOI] [PubMed] [Google Scholar]
- 33.Savolainen S, Paljarvi L, Vapalahti M. Prevalence of Alzheimer's disease in patients investigated for presumed normal pressure hydrocephalus: a clinical and neuropathological study. Acta Neurochir (Wien) 1999141849–853. [DOI] [PubMed] [Google Scholar]
- 34.Boller F, Lopez O L, Moossy J. Diagnosis of dementia: clinicopathologic correlations. Neurology 19893976–79. [DOI] [PubMed] [Google Scholar]
- 35.Dekosky S T, Harbaugh R E, Schmitte F A.et al Cortical biopsy in Alzheimer's disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Ann Neurol 199232625–635. [DOI] [PubMed] [Google Scholar]
- 36.Price J L, Davis P B, Morris J C.et al The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 199112295–312. [DOI] [PubMed] [Google Scholar]
- 37.Klinge P, Marmarou A, Bergsneider M.et al Outcome of shunting in idiopathic normal‐pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery 200557S40–S52. [DOI] [PubMed] [Google Scholar]
- 38.Albeck M J, Skak C, Nielsen P R.et al Age dependency of resistance to cerebrospinal fluid outflow. J Neurosurg 199889275–278. [DOI] [PubMed] [Google Scholar]
- 39.Czosnyka M, Czosnyka Z H, Whitfield P C.et al Age dependence of cerebrospinal pressure‐volume compensation in patients with hydrocephalus. J Neurosurg 200194482–486. [DOI] [PubMed] [Google Scholar]
- 40.Boon A J, Tans J T, Delwel E J.et al Dutch Normal‐Pressure Hydrocephalus Study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg 199787687–693. [DOI] [PubMed] [Google Scholar]
- 41.Marmarou A, Bergsneider M, Klinge P.et al The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal‐pressure hydrocephalus. Neurosurgery 200557S17–S28. [DOI] [PubMed] [Google Scholar]
- 42.Bech‐Azeddine R, Waldemar G, Knudsen G M.et al Idiopathic normal‐pressure hydrocephalus: evaluation and findings in a multidisciplinary memory clinic. Eur J Neurol 20018601–611. [DOI] [PubMed] [Google Scholar]
- 43.Bech‐Azeddine R, Gjerris F, Waldemar G.et al Intraventricular or lumbar infusion test in adult communicating hydrocephalus? Practical consequences and clinical outcome of shunt operation. Acta Neurochir (Wien) 20051471027–1036. [DOI] [PubMed] [Google Scholar]
- 44.Tullberg M, Hultin L, Ekholm S.et al White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand 2002105417–426. [DOI] [PubMed] [Google Scholar]
- 45.Skoog I. Status of risk factors for vascular dementia. Neuroepidemiology 1998172–9. [DOI] [PubMed] [Google Scholar]
- 46.Skoog I. The interaction between vascular disorders and Alzheimer's disease. In: Ikbal K, ed. Alzheimer's disease and related disorders: etiology, pathogenesis and therapeutics. New York: Wiley, 1999