Abstract
Background
Previous studies have found the e4 allele of the apolipoprotein E gene (APOE e4) is associated with an unfavourable outcome after head injury, but this has not been related to specific pathological features.
Objectives
This study tested the postulate that head injured patients with APOE e4, amounting to approximately a third of the population, are selectively predisposed to one or more of the different pathological features that constitute the response to traumatic brain injury (TBI), and that this underlies the association of APOE e4 with poor clinical outcome.
Methods
Included in the study were 239 fatal cases of TBI (1987–1999) for which APOE genotypes were determined from archival tissue. For each case, specific pathological features of trauma were recorded by researchers blinded to the APOE e4 status. Of the 239 cases examined, 83 (35%) were APOE e4 carriers and 156 (65%) were non‐carriers.
Results
Possession of APOE e4 was associated with a greater incidence of moderate or severe contusions (42% v 30% for carriers versus e4 non‐carriers; p = 0.05) and there was a trend towards a greater incidence of severe ischaemic brain damage (54% v 42%; p = 0.08). Significant differences were not noted between the other pathological features examined.
Conclusions
Possession of APOE e4 is associated with a greater incidence of moderate/severe contusional injury and severe ischaemic brain damage in fatal cases of TBI. This may be relevant to the relatively poor outcome from traumatic brain injury in patients with APOE e4 identified in clinical studies.
Keywords: APOE, polymorphisms, head injury, contusions, ischaemic damage
Clinical studies of traumatic brain injury (TBI) have shown that possession of the e4 allele of the apolipoprotein E gene (APOE e4) is associated with a relatively poor outcome.1,2 In one such study, 57% of APOE e4 carriers had an unfavourable outcome (defined as dead, in a vegetative state, or with severe disability) compared with 27% of non‐carriers of APOE e4.2 There is evidence that APOE e4 carriers also have worse outcome after spontaneous intracerebral haemorrhage,3,4 cardiac bypass surgery,5,6 cerebral ischaemia after cardiopulmonary resuscitation,7 and in boxing.8 In subarachnoid haemorrhage the evidence is conflicting,9,10,11 and the effect appears not to influence outcome from ischaemic stroke.12
The specific mechanisms by which APOE genotype influences outcome after brain injury in humans are largely unclear. Much of the work relating to mechanisms involving APOE has been undertaken using in vitro cell cultures and animal models, and the direct relevance of these studies to man is uncertain.13 Relevant mechanisms postulated range from basic cellular functions such as maintenance of cytoskeletal integrity,14 and protection from oxidative stress15 and excitotoxcicity,16 to general systemic dysfunction such as increased risk of atherosclerosis17 and altered blood coagulation.18
The pathology of TBI can be classified as either focal or diffuse.19 Focal injuries include contusions, intracranial haemorrhages, and the vascular complications of raised intracranial pressure. Diffuse injuries include diffuse traumatic axonal injury (TAI), cerebral swelling, and ischaemic brain damage. Multiple factors influence the type and severity of the resulting brain injury, and include the mechanism, location, and magnitude of the primary injury, and host factors such as age and nutritional status.
We postulate that head injured patients with APOE e4, amounting to approximately a third of the population, are selectively predisposed to one or more of the different pathological features that constitute the response to TBI, and that this underlies the association of APOE e4 with poor clinical outcome. We sought to test this hypothesis by identifying the prevalence of specific pathological features in APOE e4 carriers, compared with non‐carriers of APOE e4, in a large tissue and data archive of fatal cases of head injury.
METHODS
Case selection
The study was approved by the research ethics committee of the Southern General Hospital, Glasgow. Cases were selected from the archive of paraffin embedded tissue of the Glasgow Neuropathology department. Initially, tissue samples within the archive from all patients who died from TBI during the 13 year period 1987–1999, which were examined by us, were selected. While many of the cases had been managed by the Department of Neurosurgery, Institute of Neurological Sciences, some patients had died at district general hospitals or at the scene of the incident. Thus, 259 cases were identified (age range 2 months to 89 years). A prerequisite for inclusion in this study was successful APOE genotyping from the formalin fixed, paraffin embedded, postmortem brain tissue.
APOE genotyping
APOE genotype was determined using a previously described PCR method.20 Of the initial 259 cases, 239 were successfully genotyped (92% success rate). Unsuccessful cases were discarded after four attempts at genotyping.
Pathological data
Archival data that had been gathered prospectively during the years 1987–1999 inclusive, according to a uniform protocol, were logged into a database and included the following information for each case: age, length of survival after episode of TBI, skull fractures, intracranial haemorrhages, diffuse traumatic axonal injury (TAI), ischaemic brain damage, raised intracranial pressure and associated infarcts, and contusions.
Skull fractures were documented as being either present or absent, and intracranial haemorrhages were recorded in relation to the anatomical compartment involved (extradural, subdural, intracerebral).
TAI was documented as being absent or present, and if present was graded as grade 1 (widespread axonal damage in the corpus callosum, the cerebral hemispheres, and the brainstem), 2 (as for grade 1, plus focal haemorrhagic lesions in the corpus callosum) or 3 (as for grade 2, plus haemorrhagic lesion in the rostral brain stem).21 The term diffuse axonal injury (DAI) was originally applied to traumatic damage exclusively; however, as immunohistochemical studies using β‐amyloid precursor protein (APP) as a marker of axonal damage have demonstrated, many brain insults can result in axonal damage. Therefore, it has been proposed that that the aetiology of any axonal damage should always be indicated, and that DAI (as originally defined) now be referred to as TAI.22
Ischaemic brain damage was assessed using a grading system: severe if the lesions were diffuse, multifocal, and large within arterial territories; moderate if the lesions were limited to the arterial boundary zones, singly or in combination with subtotal infarction in the distribution of the cerebral arteries, or if there were 6–10 subcortical lesions; and mild if there were ⩽5 subcortical lesions in the brain.23
Raised intracranial pressure was considered to be present if there were tentorial hernias (either macroscopic or microscopic),24 and associated vascular complications within the distributions of the anterior cerebral artery, the posterior cerebral artery, and in the cerebellum and brainstem.
Contusions were graded using the total contusion index (TCI) developed by Adams et al,25 and subsequently modified.26 This assesses the extent (0–3) and depth (0–4) of contusions in a variety of anatomical locators, producing a numerical score for each hemisphere, which is then combined and interpreted as absent, mild, moderate, or severe. The anatomical locators are the frontal, temporal, parietal and occipital lobes, the cortex above and below the Sylvian fissure, and the cerebellum. The maximum score for an anatomical locator is 12 (4×3 = 12), and the TCI has a maximum value of 144 (each side 6×12 = 72, 2×72 = 144). For this study, contusional injury was mild if the TCI was <20, moderate if the TCI was between 20 and 37, and severe if the TCI was >37. These values were based on those used in previous studies.27
Data analysis
Eight different pathological features were considered individually. The pathological features for each case documented on the database were then assessed in relation to APOE e4 allele carriage presence or absence (table 1). Comparison of the prevalence of the features was made using confidence intervals (CI) for the differences in proportions. Calculations were performed using Minitab (version 12).
Table 1 Differences between APOE e4 carriers and non‐carriers .
| Pathological feature | Carriers (n = 83; 35%) | Non‐carriers (n = 156; 65%) | 95% CI for difference | p | ||||
|---|---|---|---|---|---|---|---|---|
| Moderate/severe contusions | 35 (42%) | 46 (29%) | 0 to 25% | 0.05 | ||||
| Severe ischaemic brain damage | 45 (54%) | 66 (42%) | −1 to 25% | 0.08 | ||||
| Skull fracture | 61 (73%) | 105 (67%) | −6 to 18% | 0.31 | ||||
| Traumatic axonal injury | 31 (37%) | 68 (44%) | −19 to 7% | 0.35 | ||||
| Extradural haemorrhage | 13 (16%) | 13 (8%) | −4 to 14% | 0.31 | ||||
| Subdural haemorrhage | 52 (60%) | 98 (63%) | −13 to 13% | 0.98 | ||||
| Intracerebral haemorrhage | 31 (37%) | 47 (30%) | −5 to 20% | 0.26 | ||||
| Raised intracranial pressure | 58 (70%) | 98 (63%) | −5 to 20% | 0.27 |
RESULTS
Of the 239 cases of fatal TBI examined, 83 were APOE e4 carriers (35%) and 156 were non‐carriers (65%). Differences were noted between APOE e4 carriers and non‐carriers of APOE e4 in relation to contusions and ischaemic brain damage (table 1).
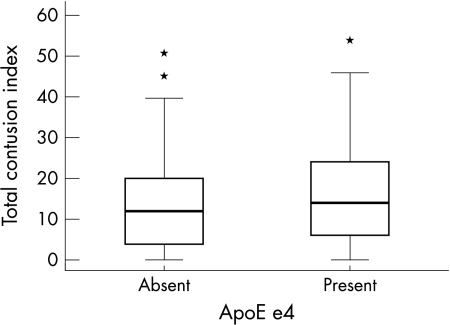
The distribution of total contusion index against APOE genotype is illustrated in a box plot (fig 1); 42% of e4 carriers (35/83) had moderate or severe contusions (that is, a TCI of >20) compared with 29% of non‐carriers of e4 (46/156, p = 0.05). Therefore, while contusions in themselves were not seen more frequently between groups, they were more likely to be of greater severity when they were present. The distribution of contusions between APOE e4 heterozygotes and homozygotes is outlined in table 2. The small number of homozygotes present did not allow for statistical analysis.
Figure 1 A box plot graph showing the distribution of total contusion index (TCI) scores between the e4 carrier and non‐carrier groups. There was a significant increase in TCI >20 in the e4 carrier group.
Table 2 Distribution of contusions for APOE e4 heterozygotes and homozygotes .
| No. of copies of e4 | Heterozygotes | Homozygotes | ||||
|---|---|---|---|---|---|---|
| No contusions | 26 (17%) | 11 (15%) | 1 (10%) | |||
| Mild contusions | 84 (54%) | 33 (45%) | 3 (30%) | |||
| Moderate contusions | 43 (27%) | 25 (35%) | 5 (50%) | |||
| Severe contusions | 3 (2%) | 4 (5%) | 1 (10%) |
With regard to ischaemic brain damage, a trend was noted between the possession of APOE e4 and severe ischaemic brain damage, which was present in 54% of APOE e4 carriers and 42% of non‐carriers of e4 (p = 0.08). No significant associations were demonstrated between possession of APOE e4 and the presence of extradural haematoma, subdural haematoma, intracerebral haematoma, skull fracture, traumatic axonal injury, or evidence of raised intracranial pressure.
DISCUSSION
Among the pathological features present in fatal cases of TBI, this study has identified an association between possession of APOE e4 and contusion severity and a trend for an association with severe ischaemic brain damage. These findings suggest that cerebrovascular and haematological mechanisms may underlie, at least in part, the association of APOE e4 with poor outcome after TBI. A limitation of this study relates to the fact that the pathology of only the most severe outcome from TBI group could be assessed (that is, a fatal outcome). In addition, this study is purely observational, based only on pathological assessment of injuries, and does not take into account any possible changes in neurosurgical referral and treatment practice which may have occurred over the 13 year period. The cases studied had variable mechanisms of injury (road traffic accidents, fall, assault) which would result in variable forces being applied to the head, and a wide range of ages; however, APOE e4 was distributed across all the various types of injury and ages.
A fatal outcome occurs in only 5–10% of the total hospitalised TBI population, although approximately 50% of TBI related deaths occur before the patient can be transferred to hospital.28 An important question is whether the association identified in this study is of relevance to survivors of TBI. A recent study, performed in the same institute, of computed tomography scans of survivors of TBI29 showed that although patients with APOE e4 were no more likely to have intracranial haemorrhages than non‐carriers, the haemorrhages were of greater volume, if they were present, in those patients with APOE e4. This study, therefore, provides a degree of clinical correlation with our post‐mortem examination based work, and suggests that our findings may well be relevant to survivors of TBI.
Although prospective clinical studies of outcome after TBI have identified APOE e4 carriers as more likely to fall into poor outcome or poor recovery groups,1,2 they have not yet specifically addressed the question of whether APOE e4 carriers are more likely to have a fatal outcome. The previous clinical studies have looked at the prevalence e4 carriers in poor outcome (severe, vegetative state, or fatal) after TBI2 or in patients in a vegetative state only.1 The present study, looking at only the fatal outcome group, did not find an over‐representation of e4 carriers, the APOE e4 carriage rate (35%) being similar to that of all head injured patients admitted to the same institution (33%, n = 984, Teasdale et al, unpublished observations). Therefore, although APOE e4 associated vascular pathology may influence the outcome in survivors, it seems unlikely to significantly increase the probability of a fatal outcome after TBI. However, the situation after spontaneous intracerebral haemorrhage appears to be different; there is evidence that among patients with stroke due to spontaneous intracerebral haemorrhage, APOE e4 carriers are substantially more likely to die in hospital (40% v 25%).30
These findings point towards an important role for APOE in cerebrovascular and haematological mechanisms that are of relevance in the response to an episode of brain injury. More specifically, existing evidence indicates that APOE may play an important role in relation to both blood vessel wall integrity and coagulation of blood. One role of APOE is as a lipid transport protein, and it is therefore involved in the transport of the fat soluble vitamins in association with lipids from the small intestine to the liver. This mechanism is suggested to underlie the relatively low levels of plasma vitamin K in APOE e4 carriers.31 Vitamin K is required by the liver for the synthesis of clotting factors, and prothrombin times have been reported to vary with APOE genotype.32 Prolonged clotting times were also identified in APOE e4 carriers after stroke,18 providing further evidence that APOE genotype is of relevance to the coagulation cascade. The possibility that contusions in head injured patients with APOE e4 are more severe as a result of relatively deficient clotting mechanisms provides the basis for a testable hypothesis.
A further mechanism of possible relevance to the findings of this study relates to the increased prevalence of atherosclerosis and cerebral amyloid angiopathy in carriers of APOE e4. Such vascular pathology might pre‐date the head injury and promote contusional haemorrhage by increasing vascular fragility and decreasing the capacity for reactive vasoconstriction. Post‐mortem studies have confirmed the association of APOE e4 with cerebral amyloid angiopathy in patients who have died from TBI, and have suggested that this is associated with increased severity of contusions,33 although the number of cases in this study was small.
Animal models, using APOE knockout and transgenic mice, have provided further information about APOE mechanisms and the response of the brain to injury.13 APOE deficient mice were found to have larger infarcts than wild type mice34 and a greater extent of neuronal damage after controlled ischaemia,35 which can be ameliorated by continuous intracerebral infusion of APOE.36 Using transgenic mice, differences have been demonstrated between the response to ischaemia and excitotoxicity in mice with human APOE e3 and APOE e4 genes, such that the APOE e4 mice have larger lesions37,38 than APOE e3 mice.39
Further elucidation of potential vascular and haematological mechanisms that may underlie the role of APOE in response to brain injury could result in the development of new therapeutic interventions which may modify the outcome after TBI.
ACKNOWLEDGEMENTS
This work was funded in part by the Medical Research Council (G9814486). C Smith was supported by a Clinical Research Fellow grant from the Scottish Council for Postgraduate Medical and Dental Education, UK.
Abbreviations
APP - beta‐amyloid precursor protein
DAI - diffuse axonal injury
TAI - traumatic axonal injury
TBI - traumatic brain injury
TCI - total contusion index
Footnotes
Competing interests: none
References
- 1.Sorbi S, Nacmias N, Piacentini S.et al ApoE as a prognostic factor for post‐traumatic coma. Nat Med 19951852. [DOI] [PubMed] [Google Scholar]
- 2.Teasdale G M, Nicoll J A, Murray G.et al Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 19973501069–1071. [DOI] [PubMed] [Google Scholar]
- 3.Alberts M J, Graffagnino C, McClenny C.et al ApoE genotype and survival from intracerebral haemorrhage. Lancet 1995346575. [DOI] [PubMed] [Google Scholar]
- 4.McCarron M O, Hoffmann K L, DeLong D M.et al Intracerebral hemorrhage outcome: apolipoprotein E genotype, hematoma, and edema volumes. Neurology 1999532176–2179. [DOI] [PubMed] [Google Scholar]
- 5.Newman M F, Croughwell N D, Blumenthal J A.et al Predictors of cognitive decline after cardiac operation. Ann Thorac Surg 1995591326–1330. [DOI] [PubMed] [Google Scholar]
- 6.Tardiff B E, Newman M F, Saunders A M.et al Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg 199764715–720. [DOI] [PubMed] [Google Scholar]
- 7.Schiefermeier M, Kollegger H, Madl C.et al Apolipoprotein E polymorphism: survival and neurological outcome after cardiopulmonary resuscitation. Stroke 2000312068–2073. [DOI] [PubMed] [Google Scholar]
- 8.Jordan B D, Relkin N R, Ravdin L D.et al Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 1997278136–140. [PubMed] [Google Scholar]
- 9.Niskakangas T, Ohman J, Niemela M.et al Association of apolipoprotein E polymorphism with outcome after aneurysmal subarachnoid hemorrhage: a preliminary study. Stroke 2001321181–1184. [DOI] [PubMed] [Google Scholar]
- 10.Dunn L T, Stewart E, Murray G D.et al The influence of apolipoprotein E genotype on outcome after spontaneous subarachnoid hemorrhage: a preliminary study. Stroke 200233548–552. [DOI] [PubMed] [Google Scholar]
- 11.Leung C H, Poon W S, Yu L M.et al Apolipoprotein E genotype and outcome in aneurysmal subarachnoid hemorrhage. Stroke 200233548–552. [DOI] [PubMed] [Google Scholar]
- 12.McCarron M O, Muir K W, Nicoll J A.et al Prospective study of apolipoprotein E genotype and functional outcome following ischemic stroke. Arch Neurol 2000571480–1484. [DOI] [PubMed] [Google Scholar]
- 13.Horsburgh K, McCarron M O, White F.et al The role of apolipoprotein E in Alzheimer's disease, acute brain injury and cerebrovascular disease: evidence of common mechanisms and utility of animal models. Neurobiol Aging 200021245–255. [DOI] [PubMed] [Google Scholar]
- 14.Roses A D, Einstein G, Gilbert J.et al Morphological, biochemical, and genetic support for an apolipoprotein E effect on microtubular metabolism. Ann N Y Acad Sci 1996777146–157. [DOI] [PubMed] [Google Scholar]
- 15.Lomnitski L, Kohen R, Chen Y.et al Reduced levels of antioxidants in brains of apolipoprotein E‐deficient mice following closed head injury. Pharmacol Biochem Behav 199756669–673. [DOI] [PubMed] [Google Scholar]
- 16.Tolar M, Keller J N, Chan S.et al Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. J Neurosci 1999197100–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hixson J E. Apolipoprotein E polymorphisms affect atherosclerosis in young males. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb 1991111237–1244. [DOI] [PubMed] [Google Scholar]
- 18.Weir C J, McCarron M O, Muir K W.et al Apolipoprotein E genotype, coagulation, and survival following acute stroke. Neurology 2001571097–1100. [DOI] [PubMed] [Google Scholar]
- 19.Graham D I, Gennarelli T A, McIntosh T K. Trauma. In: Graham DI, Lantos PL, eds. Greenfield's neuropathology. 7th ed. London: Arnold, 2002823–898.
- 20.Nicoll J A, Burnett C, Love S.et al High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol 199741716–721. [DOI] [PubMed] [Google Scholar]
- 21.Adams J H, Doyle D, Ford I.et al Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 19891549–59. [DOI] [PubMed] [Google Scholar]
- 22.Geddes J F, Whitwell H L, Graham D I. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol Appl Neurobiol 200026105–116. [DOI] [PubMed] [Google Scholar]
- 23.Graham D I, Ford I, Adams J H.et al Ischaemic brain damage is still common in fatal non‐missile head injury. J Neurol Neurosurg Psychiatry 198952346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams J H, Graham D I. The relationship between ventricular fluid pressure and the neuropathology of raised intracranial pressure. Neuropathol Appl Neurobiol 19762323–332. [Google Scholar]
- 25.Adams J H, Graham D I, Scott G.et al Brain damage in fatal non‐missile head injury. J Clin Path 1980331132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams J H, Doyle D, Graham D I.et al The contusion index: a reappraisal in human and experimental non‐missile head injury. Neuropathol Appl Neurobiol 198511299–308. [DOI] [PubMed] [Google Scholar]
- 27.Graham D I, Lawrence A E, Adams J H.et al Brain damage in fatal non‐missile head injury without high intracranial pressure. J Clin Pathol 19884134–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennett B, MacMillan R Epidemiology of head injury BMJ. 1981;282:101–104. doi: 10.1136/bmj.282.6258.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liaquat I, Dunn L T, Nicoll J A.et al Effect of apolipoprotein E genotype on hematoma volume after trauma. J Neurosurg 20029690–96. [DOI] [PubMed] [Google Scholar]
- 30.McCarron M O, Weir C J, Muir K W.et al Effect of apolipoprotein E genotype on in‐hospital mortality following intracerebral haemorrhage. Acta Neurol Scand 2003107106–109. [DOI] [PubMed] [Google Scholar]
- 31.Shearer M J. Vitamin K. Lancet 1995345229–234. [DOI] [PubMed] [Google Scholar]
- 32.Giraud V, Naveau S, Betoulle D.et al Influence of apolipoprotein E polymorphism in alcoholic cirrhosis. Gastroenterol Clin Biol 199822571–575. [PubMed] [Google Scholar]
- 33.Leclercq P D, Murray L S, Smith C.et al Cerebral amyloid angiopathy in traumatic brain injury: association with apolipoprotein E genotype. J Neurol Neurosurg Psychiatry 200576229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laskowitz D T, Sheng H, Bart R D.et al Apolipoprotein E‐deficient mice have increased susceptibility to focal cerebral ischemia. J Cereb Blood Flow Metab 199717753–758. [DOI] [PubMed] [Google Scholar]
- 35.Horsburgh K, Kelly S, McCulloch J.et al Increased neuronal damage in apolipoprotein E‐deficient mice following global ischaemia. Neuroreport 199910837–841. [DOI] [PubMed] [Google Scholar]
- 36.Horsburgh K, McCulloch J, Nilsen M.et al Intraventricular infusion of apolipoprotein E ameliorates acute neuronal damage after global cerebral ischemia in mice. J Cereb Blood Flow Metab 200020458–462. [DOI] [PubMed] [Google Scholar]
- 37.Horsburgh K, McCulloch J, Nilsen M.et al Increased neuronal damage and apoE immunoreactivity in human apolipoprotein E, E4 isoform‐specific, transgenic mice after global cerebral ischaemia. Eur J Neurosci 2000124309–4317. [PubMed] [Google Scholar]
- 38.Buttini M, Orth M, Bellosta S.et al Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform‐specific effects on neurodegeneration. J Neurosci 1999194867–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buttini M, Akeefe H, Lin C.et al Dominant negative effects of apolipoprotein E4 revealed in transgenic models of neurodegenerative disease. Neuroscience 200097207–210. [DOI] [PubMed] [Google Scholar]