Abstract
We report the case of a 28 year old man who had received a cadaverous dura mater graft after a traumatic open skull fracture with tearing of the dura at the age of 5 years. A clinical suspicion of Creutzfeldt‐Jakob disease (CJD) was confirmed by a brain biopsy 5 months prior to death and by autopsy, thus warranting the diagnosis of iatrogenic CJD (iCJD) according to WHO criteria. Immunohistochemistry showed widespread cortical depositions of disease associated prion protein (PrPsc) in a synaptic pattern, and western blot analysis identified PrPsc of type 2A according to Parchi et al. Surprisingly, we found Alzheimer‐type senile plaques and cerebral amyloid angiopathy in widespread areas of the brain. Plaque‐type and vascular amyloid was immunohistochemically identified as deposits of beta‐A4 peptide. CERAD criteria for diagnosis of definite Alzheimer's disease (AD) were met in the absence of neurofibrillar tangles or alpha‐synuclein immunoreactive inclusions. There was no family history of AD, CJD, or any other neurological disease, and genetic analysis showed no disease specific mutations of the prion protein, presenilin 1 and 2, or amyloid precursor protein genes. This case represents (a) the iCJD case with the longest incubation time after dural grafting reported so far, (b) the youngest documented patient with concomitant CJD and Alzheimer‐type neuropathology to date, (c) the first description of Alzheimer‐type changes in iCJD, and (d) the second case of iCJD in Austria. Despite the young patient age, the Alzheimer‐type changes may be an incidental finding, possibly related to the childhood trauma.
Keywords: Alzheimers disease, trauma, iatrogenic Creutzfeldt‐Jakob disease
Creutzfeldt‐Jakob disease (CJD) and Alzheimer's disease (AD) are neurodegenerative diseases that share some clinical, neuropathological, and pathogenic features.6,8,10,21,32 AD may occur sporadically or as inherited disorder and is considered a non‐transmissible disease. CJD may be acquired by inoculation of infectious material during therapeutic interventions, such as dura mater transplantation or growth hormone substitution (iatrogenic CJD or iCJD).4 Amyloid accumulation is a characteristic feature of both CJD and AD. It is composed of abnormally conformed prion protein (PrPsc) in CJD and of beta‐amyloid (BA) in AD. BA may deposit as plaques in the brain parenchyma or in vessel walls in AD.29,33 There are several previous reports about Alzheimer‐type changes in elderly sporadic CJD patients.1,3,12,13,20,22,25,27 We report here the presence of plaque‐type and vascular BA deposits in a 28 year old patient with iCJD who had undergone a post‐traumatic dura transplantation 23 years before death. This case represents (a) the iCJD case with the longest incubation time after dural grafting reported so far, (b) the youngest documented patient with concomitant CJD and Alzheimer‐type neuropathology to date, (c) the first description of Alzheimer‐type changes in iCJD, and (d) the second case of iCJD in Austria.28
CASE REPORT
Clinical history
In 1982, a normally developed 5 year old boy suffered a traumatic open left frontobasal depressed skull fracture with left frontal haemorrhagic brain contusion and tearing of the dura mater. In addition, there was a comminuted fracture of the left orbital wall. Post‐trauma, the patient was unconscious but responded to painful stimuli. Intracranial hypertension or hypoxic brain lesions were not recorded in the patient history. The dural defect was surgically covered using lyophilised cadaveric dura. Reconstructive surgery of the skull was performed using autologous costal bone. There was good recovery from the accident, and further cognitive and social development was normal.
In December 2003, the patient began to develop progressive neurological symptoms with personality changes, myoclonus, dementia, and seizures. There were no cerebellar symptoms. Several electroencephalographic examinations showed various pathological changes, including generalised slowing, burst suppression pattern, and periodic sharp waves. The 14‐3‐3 protein was detectable in the cerebrospinal fluid (CSF). Computer tomography showed a large cystic defect of the left frontal lobe and residuals of the skull fracture. Magnetic resonance imaging suggested discrete hyperintensities in the caudate nucleus and the putamen in T2 weighted sequences. In addition, neuroimaging showed progressive dilatation of the third ventricle and the anterior horns, suggesting impaired CSF circulation. Clinically, there was a differential diagnosis between CJD and prolonged epileptic status. A diagnostic brain biopsy of the left frontal lobe was performed 6 months after disease onset to clarify this differential diagnosis, which yielded the diagnosis of definite CJD (see below). The diagnosis of iCJD was made according to the WHO definition.
Following the biopsy, there was CSF leakage from the wound, which was treated by pressure bandage. In the last months before death, the patient developed relapsing bacteraemia and urinary tract infections that were treated with antibiotics. The patient died after a disease duration of 8 months. There was no family history of dementia or any other neurological disease.
Neuropathology
The biopsy material showed slight gliosis and marked spongiform change without prominent nerve cell loss. Immunohistochemically, depositions of PrPsc in a diffuse synaptic pattern were demonstrated using four different antibodies (12F10, 3F4, 6H4, KG9; table 1). Immunohistochemistry using an anti‐BA antibody (6F/3D; table 1) revealed single plaque‐type depositions and amyloid deposition in the wall of some vessels. Bielschowsky staining showed single neuritic plaques. There were no tau (AT8; table 1) or alpha‐synuclein (15G7; table 1) immunoreactive inclusion bodies.
Table 1 Summary of antibodies and immunostaining methods used in this study.
| Antibody | Specificity | Supplier | Pretreatment | Dilution | Incubation time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12F10 | Prion protein | Cayman, Ann Arbor, MI, USA | D. H2O 30 min at 121°C + 5 min FA | 1:1000 | 1 hour | |||||
| 3F4 | Prion protein | Signet, Dedham, MA, USA | D. H2O. 30 min at 121°C + 5 min FA | 1:500 | 1 hour | |||||
| 6H4 | Prion protein | Prionics, Schlieren, Switzerland | D. H2O. 30 min at 121°C + 5 min FA | 1:500 | 1 hour | |||||
| KG9 | Prion protein | TSE Resource Center, Newbury, UK | D. H2O 30 min at 121°C + 5 min FA | 1:1000 | 1 hour | |||||
| 6F/3D | N‐terminus beta‐amyloid | Dako Cytomation, Glostrup, Denmark | 2 min FA | 1:50 | 25 min | |||||
| A‐Beta1‐40 | C‐terminus A‐beta1‐40 | Signet, Dedham, MA, USA | 30 min FA | 1:100 | 32 min | |||||
| A‐Beta1‐42 | C‐terminus A‐beta1‐42 | Signet, Dedham, MA, USA | 30 min FA | 1:100 | 32 min | |||||
| AT8 | Tau | Pierce Endogen, Rockford, IL, USA | No pretreatment | 1:200 | 25 min | |||||
| 15G7 | Alpha‐synuclein | Alexis Biochemicals, Lausen, Switzerland | AR 10 min in citrate buffer (pH 6.0) + 1 min FA | 1:20 | 1 hour |
D. H20, distilled water; AR, antigen retrieval, FA formic acid.
At autopsy, the patient's brain weight was 974 g. Macroscopically, the brain showed prominent internal hydrocephalus. There were large cystic defects at the left frontobasis with connection to the left lateral ventricle. There was only slight thinning of the cerebral and cerebellar cortical ribbon. The Ammon's horn and substantia nigra were inconspicuous. There was slight thickening of the basal meninges.
Histologically, there was the classical triad of CJD with severe gliosis, nerve cell loss, and spongiform neuropil alteration in the cerebral and the cerebellar cortices (fig 1A). A few eosinophilic plaques were noted in haematoxylin and eosin stained samples from the cerebral cortex. They were congophilic and showed green bi‐refringence in polarised light. In the left frontal lobe, there were residuals of the old traumatic contusion, with glial scarring, cystic alteration, and old blood pigment. In addition, there was a partly cystic old infarct with glial scarring in the left temporal cortex. There was mononuclear granuloma‐like inflammation around bony particles in the area of the brain biopsy in the left frontal lobe. In addition, there was prominent and widespread ventriculitis and meningitis with mononuclear inflammatory infiltrates. Gram, Gram‐Twort, periodic acid Schiff, Ziehl‐Neelsen, and Fite stains did not show microorganisms. These inflammatory changes may have occurred due to the post‐bioptic CSF leakage.
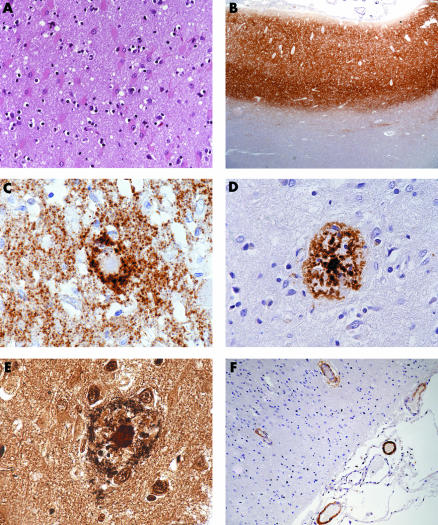
Figure 1 (A) Prominent gliosis, nerve cell loss, and spongiform change in the cerebral cortex (haematoxylin and eosin, ×200). (B) Widespread diffuse synaptic deposits of disease‐associated prion protein in the cerebral cortex (12F10, ×20). (C) Plaques do not show immunostaining for PrPsc but show peripheral wreath‐like accumulation of PrPsc (12F10, 600×). (D) Plaques show immunostaining for beta‐amyloid (anti‐beta‐A4, ×400). (E) Bielschowsky staining identifies neuritic plaques in the cerebral cortex (Bielschowsky, ×600). (F) Some meningeal and parenchymal vessels show depositions of beta‐amyloid in their wall (anti‐beta‐A4, ×600).
Immunohistochemistry showed diffuse “synaptic” deposits of PrPsc in all cerebral and cerebellar cortical areas (fig 1B) and the basal ganglia including putamen and caudate nucleus; there were no plaque‐like deposits of PrPsc. There were anti‐BA immunoreactive amyloid plaques (fig 1D) in the frontal, temporal, parietal, occipital, and insular cortices of both hemispheres, which often showed peripheral wreath‐like accumulation of PrPsc (fig 1C). In contrast to previous observations in Gerstmann‐Straussler‐Scheinker disease, we did not observe deposition of PrPsc in cores of BA‐plaques.5,24 Antibodies specific for A‐beta1‐40 labelled peripheral portions of plaque cores while sparing core centres. Antibodies against A‐beta1‐42 labelled peripheral portions and cores of BA plaques.
We manually assessed the BA burden in an area of 1 mm2 containing the maximal BA load in five cortical areas using an eye grid. The percentage of the area occupied by A‐beta1‐42 or A‐beta1‐40 was 2.8% or 0.25% in the cingular cortex, 0.25% or 0.06% in the occipital cortex, 2.2% or 0.22% in the frontal cortex, 1.5 % or 0.75% in the temporal cortex, and 1.2% or 0.15% in the parietal cortex, respectively. Both Ammon's horns, the striatum of both hemispheres, midbrain, pons, medulla oblongata, and cerebellar cortex were devoid of BA plaques. Bielschowsky staining distinguished diffuse and neuritic plaques (fig 1E). Diffuse plaques showed anti‐BA immunoreactivity but lacked congophilia, thus showing the tinctorial properties of preamyloid. Using the CERAD definition,23 a “moderate” number of neuritic plaques was found in the fontal cortex of both hemispheres and “sparse” plaques were present in the temporal, parietal, occipital, and insular cortices. These findings warranted a diagnosis of “definite AD” according to CERAD criteria.23 In addition, anti‐BA immunohistochemistry identified moderate amyloid angiopathy of parenchymal and meningial vessels (fig 1F) in widespread areas of the brain including all cortical areas, both Ammon's horns, the right striatum, and the pons. Vascular BA deposits were immunolabelled with antibodies against A‐beta1‐40 and A‐beta1‐42. There were no phosphorylated tau or alpha‐synuclein immunoreactive inclusion bodies. Owing to the lack of neurofibrillary tangles, the diagnostic criteria for none of the categories of the consensus recommendations for diagnosis of AD of The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease were met.15
Genetics
Genetic analyis of presenilin 2 (PSEN2) exons 1 to 12 showed exchange of cytidine by thymidine at positions 69, 129, and 261 in one allele of PSEN2 compared with the reference sequence (OMIM 600759). These polymorphisms have been reported as having no pathogenic impact.7 Amyloid precursor protein (APP) exons 16 and 17 and presenilin 1 (PSEN1) exons 1 to 12 showed no deviations from the reference sequences (OMIM 104311 and 104760, respectively).
The prion protein gene (PRNP) showed neither a known pathogenic mutation nor a novel missense mutation in the coding region, thus an inherited prion disease was excluded. The patient was homozygous for methionine at the polymorphic codon 129 of PRNP.
Western blot
Immunoblotting of PrPsc performed on frozen tissue taken from cerebellar cortex identified PrPsc type 2A according to the classification by Parchi et al.26
DISCUSSION
The incubation time of 23 years is the longest one reported for dural graft associated iCJD so far. The median incubation time of iCJD after dural grafting is 6 years,4 and the longest incubation time reported so far was 20 years.19 Our case shows that iCJD still occurs as a result of dura mater grafts manufactured and distributed before the mid‐1980s.
There are several previous reports about Alzheimer‐type changes in CJD patients1,3,12,13,20,22,25,27 and speculations about pathogenic connections regarding amyloid accumulation between the two conditions have been raised.6,8,10 However, owing to the preponderance of both diseases in the elderly, their coexistence in some patients may represent an age related phenomenon.13 In addition, there is also rare occurrence of Alzheimer‐type changes in the age group of 26–30 years.2 The distribution of BA deposits in our patient is analogous to the distribution pattern described in early AD stages.2 Hence, despite the young patient age, the Alzheimer‐type changes may be an incidental finding in our case. Head injury has been described as a risk factor for development of dementia and Alzheimer‐type neuropathological changes.9,11,14,16,17,18,30 Therefore, BA accumulation may have been initiated by the brain injury our patient has suffered 23 years prior to death. To our knowledge, there are no other documented cases of Alzheimer‐type neuropathology in young long time survivors of severe head trauma.
To date, mutations in three genes, APP, PSEN1, and PSEN2 have been identified as the most usual causative mutations in familial AD.31 None of these three genes showed disease relevant mutations in our case, making genetic AD an unlikely cause of the BA amyloidosis. Alternatively, the pathology may have been caused by an as yet unknown mutation causing cerebrovascular BA accumulation. However, there is no history of AD in the patient's family.
In summary, despite the young patient age, the Alzheimer‐type changes in this case may be an incidental finding, possibly related to the trauma our patient has suffered in childhood. Thus, our case is compatible with previous arguments that occasional Alzheimer‐type neuropathology in CJD may be coincidental.13
ACKNOWLEDGEMENTS
This work was supported by the EU QoL project TSELAB. We thank Dr J Attems, Department of Pathology, Otto Wagner Hospital, Vienna, Austria, for providing immunostaining for A‐beta1‐40 and A‐beta1‐42. We are grateful to the patient's family for their support.
Abbreviations
AD - Alzheimer's disease
BA - beta‐amyloid
CERAD - Consortium to Establish a Registry for Alzheimer's Disease
CJD - Creutzfeldt‐Jakob disease
CSF - cerebrospinal fluid
iCJD - iatrogenic Creutzfeldt‐Jakob disease
Footnotes
Competing interests: none
References
- 1.Barcikowska M, Kwiecinski H, Liberski P P.et al Creutzfeldt‐Jakob disease with Alzheimer‐type A beta‐reactive amyloid plaques. Histopathology 199526445–450. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. Frequency of stages of Alzheimer‐related lesions in different age categories. Neurobiol Aging 199718351–357. [DOI] [PubMed] [Google Scholar]
- 3.Brown P, Jannotta F, Gibbs C J., Jret al Coexistence of Creutzfeldt‐Jakob disease and Alzheimer's disease in the same patient. Neurology 199040226–228. [DOI] [PubMed] [Google Scholar]
- 4.Brown P, Preece M, Brandel J P.et al Iatrogenic Creutzfeldt‐Jakob disease at the millennium. Neurology 2000551075–1081. [DOI] [PubMed] [Google Scholar]
- 5.Bugiani O, Giaccone G, Verga L.et al Beta PP participates in PrP‐amyloid plaques of Gerstmann‐Straussler‐Scheinker disease, Indiana kindred. J Neuropathol Exp Neurol 19935264–70. [DOI] [PubMed] [Google Scholar]
- 6.Castellani R J, Perry G, Smith M A. Prion disease and Alzheimer's disease: pathogenic overlap. Acta Neurobiol Exp (Wars) 20046411–17. [DOI] [PubMed] [Google Scholar]
- 7.Cruts M, van Duijn C M, Backhovens H.et al Estimation of the genetic contribution of presenilin‐1 and ‐2 mutations in a population‐based study of presenile Alzheimer disease. Hum Mol Genet 1998743–51. [DOI] [PubMed] [Google Scholar]
- 8.DeArmond S J. Alzheimer's disease and Creutzfeldt‐Jakob disease: overlap of pathogenic mechanisms. Curr Opin Neurol 19936872–881. [DOI] [PubMed] [Google Scholar]
- 9.Fleminger S, Oliver D L, Lovestone S.et al Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 200374857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajdusek D C. Nucleation of amyloidogenesis in infectious and noninfectious amyloidoses of brain. Ann N Y Acad Sci 1994724173–190. [DOI] [PubMed] [Google Scholar]
- 11.Gentleman S M, Greenberg B D, Savage M J.et al A beta 42 is the predominant form of amyloid beta‐protein in the brains of short‐term survivors of head injury. Neuroreport 199781519–1522. [DOI] [PubMed] [Google Scholar]
- 12.Gray F, Chretien F, Cesaro P.et al Creutzfeldt‐Jakob disease and cerebral amyloid angiopathy. Acta Neuropathol (Berl) 199488106–111. [DOI] [PubMed] [Google Scholar]
- 13.Hainfellner J A, Wanschitz J, Jellinger K.et al Coexistence of Alzheimer‐type neuropathology in Creutzfeldt‐Jakob disease. Acta Neuropathol (Berl) 199896116–122. [DOI] [PubMed] [Google Scholar]
- 14.Horsburgh K, Cole G M, Yang F.et al beta‐amyloid (Abeta) 42 (43), abeta42, abeta40 and apoE immunostaining of plaques in fatal head injury. Neuropathol Appl Neurobiol 200026124–132. [DOI] [PubMed] [Google Scholar]
- 15.Hyman B T, Trojanowski J Q. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997561095–1097. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomovic M D, Uryu K, Abrahamson E E.et al Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 2004190192–203. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger K A. Head injury and dementia. Curr Opin Neurol 200417719–723. [DOI] [PubMed] [Google Scholar]
- 18.Jellinger K A, Paulus W, Wrocklage C.et al Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer's disease. Eur J Neurol 20018707–710. [DOI] [PubMed] [Google Scholar]
- 19.Kretzschmar H A, Sethi S, Foldvari Z.et al Iatrogenic Creutzfeldt‐Jakob disease with florid plaques. Brain Pathol 200313245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberski P P, Papierz W, Alwasiak J. Creutzfeldt‐Jakob disease with plaques and paired helical filaments. Acta Neurol Scand 198776428–432. [DOI] [PubMed] [Google Scholar]
- 21.Masters C L, Beyreuther K. Neuropathology of unconventional virus infections: molecular pathology of spongiform change and amyloid plaque deposition. Ciba Found Symp 198813524–36. [DOI] [PubMed] [Google Scholar]
- 22.Masters C L, Gajdusek D C, Gibbs C J., Jr The familial occurrence of Creutzfeldt‐Jakob disease and Alzheimer's disease. Brain 1981104535–558. [DOI] [PubMed] [Google Scholar]
- 23.Mirra S S, Heyman A, McKeel D.et al The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 199141479–486. [DOI] [PubMed] [Google Scholar]
- 24.Miyazono M, Kitamoto T, Iwaki T.et al Colocalization of prion protein and beta protein in the same amyloid plaques in patients with Gerstmann‐Straussler syndrome. Acta Neuropathol (Berl) 199283333–339. [DOI] [PubMed] [Google Scholar]
- 25.Muramoto T, Kitamoto T, Koga H.et al The coexistence of Alzheimer's disease and Creutzfeldt‐Jakob disease in a patient with dementia of long duration. Acta Neuropathol (Berl) 199284686–689. [DOI] [PubMed] [Google Scholar]
- 26.Parchi P, Giese A, Capellari S.et al Classification of sporadic Creutzfeldt‐Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 199946224–233. [PubMed] [Google Scholar]
- 27.Powers J M, Liu Y, Hair L S.et al Concomitant Creutzfeldt‐Jakob and Alzheimer diseases. Acta Neuropathol (Berl) 19918395–98. [DOI] [PubMed] [Google Scholar]
- 28.Radbauer C, Hainfellner J A, Gaudernak T.et al [Creutzfeldt‐Jakob disease in a dura transplant recipient: first observation in Austria]. Wien Klin Wochenschr 1998110496–500. [PubMed] [Google Scholar]
- 29.Revesz T, Ghiso J, Lashley T.et al Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol 200362885–898. [DOI] [PubMed] [Google Scholar]
- 30.Roberts G W, Gentleman S M, Lynch A.et al Beta A4 amyloid protein deposition in brain after head trauma. Lancet 19913381422–1423. [DOI] [PubMed] [Google Scholar]
- 31.Rocchi A, Pellegrini S, Siciliano G.et al Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res Bull 2003611–24. [DOI] [PubMed] [Google Scholar]
- 32.Watson C P. Clinical similarity of Alzheimer and Creutzfeldt‐Jakob disease. Ann Neurol 19796368–369. [DOI] [PubMed] [Google Scholar]
- 33.Wong C W, Quaranta V, Glenner G G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA 1985828729–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]