Abstract
Background
Extensive investigations are often performed to reveal the cause of chronic polyneuropathy. It is not known whether a restrictive diagnostic guideline improves cost efficiency without loss of diagnostic reliability.
Methods
In a prospective multicentre study, a comparison was made between the workup in patients with chronic polyneuropathy before and after guideline implementation.
Results
Three hundred and ten patients were included: 173 before and 137 after guideline implementation. In all patients, the diagnosis would remain the same if the workup was limited to the investigations in the guideline. After guideline implementation, the time to reach a diagnosis decreased by two weeks. There was a reduction of 33% in the number and costs of routine laboratory investigations/patient, and a reduction of 27% in the total number of laboratory tests/patient, despite low guideline adherence.
Conclusion
The implementation of a diagnostic guideline for chronic polyneuropathy can reduce diagnostic delay and the number and costs of investigations for each patient without loss of diagnostic reliability. Continuous evaluation strategies after guideline implementation may improve guideline adherence and cost efficiency.
Keywords: polyneuropathies, diagnosis, diagnostic tests, clinical practice guideline, guideline adherence
Polyneuropathy has an estimated incidence of 25–200/100 000 persons/year and a prevalence of up to about 5%.1,2,3,4,5 Extensive diagnostic investigations are usually performed to reveal the cause of this disease.2,6,7,8,9,10,11,12,13 This conventional approach implies a surplus of investigations, diagnostic and therapeutic delay, and high costs. A standardised or stepwise workup has been advocated,7,8,13,14,15,16,17 but the number of investigations could probably be restricted.18,19 It is not known whether a restrictive diagnostic guideline can be implemented without loss of diagnostic reliability.
Our prospective study assessed whether the implementation of a restrictive diagnostic guideline resulted in the preservation of diagnostic reliability, reduction in diagnostic delay, and improved cost efficiency.
Methods
Our study protocol was approved by the medical ethical committees of the participating hospitals.
Study design and diagnostic guideline
Our study concerned a prospective multicentre evaluation of the workup in chronic polyneuropathy before and after guideline implementation between 1999 and the end of 2002 in five general hospitals and the University Medical Centre (UMC) in Utrecht, the Netherlands.
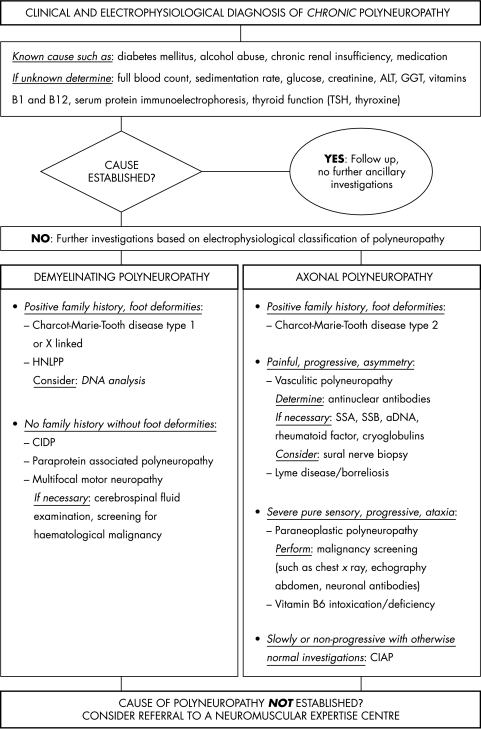
Inclusion took place in two separate periods of six months' duration with a follow up of six months for each patient. During the first inclusion period and follow up, the neurologists used their conventional diagnostic approach. For the next period, a restrictive guideline (fig 1) was handed to the neurologists after an informative meeting. Regular visits by the principal investigator (AV) to the participating hospitals for patient inclusion and data acquisition also served to remind the neurologists to use the guideline.
Figure 1 Diagnostic guideline for chronic polyneuropathy (nadir after three months). ALT, alanine aminotransferase; CIAP, chronic inflammatory axonal polyradiculoneuropathy; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; GGT, γ glutamyl transferase; HNLPP, hereditary neuropathy with liability to pressure palsies; SSA/B, Sjögren's syndrome antigen A/B; TSH, thyroid stimulating hormone.
The restrictive guideline was a modification of a previous guideline with a comprehensive list of diagnostic investigations for polyneuropathy. The modification consisted of excluding those investigations that, when carried out, did not contribute to revealing the cause of chronic polyneuropathy.18 Thus, the guideline was restricted to diagnose common causes, and did not include infrequent causes, such as folic acid and vitamin E deficiency, rare inborn metabolic diseases, Friedreich's ataxia, and other rare hereditary disorders. Because we wanted to exclude patients for whom the guideline would not apply,20,21 the guideline starts after electrophysiological confirmation of polyneuropathy, although many clinicians would order many of the investigations simultaneously. The guideline also included criteria for demyelination and conduction block used in the UMC as described elsewhere.22
Patient inclusion, data acquisition, and evaluation
Inclusion criteria were: (1) patients ⩾ 18 years old; (2) symptoms and signs of polyneuropathy with a nadir after three months; (3) electrophysiological confirmation of polyneuropathy; (4) workup in the outpatient clinic only; (5) patients in general hospitals not previously investigated by another neurologist, patients in the UMC not previously investigated in a neuromuscular centre.
Relevant data were retrieved from the medical records. The costs of the investigations were calculated with the tariffs (in Euros) issued by the college for health insurances in the Netherlands.
There were two outcome measures before and after guideline implementation. (1) Diagnostic reliability. (a) Guideline adherence, and evaluation of whether diagnoses could be retained when considering only the results of the investigations in the guideline (diagnoses by the neurologist were taken as the golden standard). (b) Agreement between the electrophysiological classification of the polyneuropathy (demyelinating or axonal) by the neurologists and that using the guideline criteria (guideline criteria for demyelination and conduction block served as the golden standard). (2) Cost efficiency. (a) Diagnostic delay: number of visits, time until diagnosis, and proportion of patients referred to a neuromuscular centre. (b) Investigations: number and costs of investigations.
Statistical analysis
The t test was used to compare normally distributed data and the Mann‐Whitney test for non‐ normally distributed data. Proportions were compared with Fisher's exact test. Two sided significance was set at p ⩽ 0.05.
Results
Six hundred and fifty and 220 patients were evaluated for chronic polyneuropathy in the five general hospitals and the UMC, respectively. A total of 310 (36%) patients met the inclusion criteria; 199 in the general hospitals (118 before and 81 after guideline implementation) and 111 in the UMC (55 before and 56 after guideline implementation).
Adherence to the guideline was low (37 of 137 patients; 27%) and most (69%) patients in whom the workup was in accordance with the guideline had diabetic polyneuropathy. About one third of patients (111 of 310; 36%) had no relevant medical history.
Diagnostic reliability
Because the frequencies of diagnoses were similar, the data (table 1) were combined for all hospitals. However, more patients with diabetic polyneuropathy (41%) were seen in the general hospitals than in the UMC (10%).
Table 1 Diagnoses for all hospitals combined, before (173 patients) and after (137 patients) guideline implementation.
| Before | After | Difference* (95% CI) | p Value | |
|---|---|---|---|---|
| Diagnosis† | ||||
| Diabetes mellitus | 32% | 26% | — | 0.21 |
| Alcohol abuse | 14% | 6% | — | 0.02 |
| Vitamin deficiency or abuse | 6% | 9% | — | 0.31 |
| Chronic renal disease | 2% | 4% | — | 0.35 |
| Medication or toxic | 2% | 3% | — | 0.70 |
| Hypothyroidism | 2% | 3% | — | 0.70 |
| Paraproteinaemia | 9% | 9% | — | 0.84 |
| Hereditary neuropathy | 3% | 7% | — | 0.29 |
| Autoimmune or systemic disease | 4% | 4% | — | 1.00 |
| CIDP | 2% | 4% | — | 0.51 |
| Malignancy | 2% | 3% | — | 0.70 |
| Idiopathic | 43% | 49% | — | 0.36 |
| Type of polyneuropathy | ||||
| Axonal | 57% | 55% | — | 0.82 |
| Demyelinating | 13% | 12% | — | 1.00 |
| Not specified‡ | 31% | 33% | — | 0.71 |
| Number of consultations/patient§ | 4 (1) | 4 (2) | 0.1 (−0.3 to 0.5) | 0.56 |
| Time to diagnosis/patient (months)§ | 3.5 (2.4) | 2.7 (2.5) | −0.4 (−1.0 to 0.1) | 0.15 |
| Repeated investigations | 30% | 34% | — | 0.54 |
CI, confidence interval; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; UMCU, University Medical Centre Utrecht.
*Mean difference for the number of consultations and the time to diagnosis; †multiple diagnoses possible; ‡all in general hospitals, not in UMCU; §mean (SD).
In 25 patients, neurologists had overlooked the following possible causes that would still have been identified by strict guideline adherence: alcohol abuse (nine patients); vitamin B6 abuse (eight patients); chronic renal disease (three patients); diabetes mellitus, hypothyroidism, and paraproteinaemia (two patients each); vitamin B12 deficiency and medication (one patient each). In 15 of these patients a diagnosis of idiopathic polyneuropathy was made. In all patients in whom a conventional workup was done, the diagnosis would not have changed if only the guideline investigations were considered.
Except for one patient, the electrophysiological investigations in the general hospitals did not meet guideline criteria. Reasons were: no control of skin temperature (95%), no electromyographic investigation of the anterior tibial muscle (40%), no conduction study of the sural nerve (24%), or no conduction studies of the arms despite the presence of symptoms (21%). Disregarding the temperature criterion, agreement between the electrophysiological classification made by the neurologists and that of the guideline increased non‐significantly: 76% before versus 86% after guideline implementation (p = 0.35). In the UMC, all electrophysiological investigations were performed according to the guideline, with 100% concordance in classification before and after guideline implementation.
Cost efficiency
Because separate analyses showed similar results, the outcome measures of cost efficiency (table 2) were combined for all hospitals.
Table 2 Number and costs of investigations for all hospitals combined, costs before (173 patients) and after (137 patients) guideline implementation.
| Test | Number of investigations/patient | Costs (in €) of investigations/patient | ||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Difference* (95% CI) | p Value | Before | After | Difference* (95% CI) | p Value | |
| Electrolytes and glucose | 3 (3) | 2 (2) | −42% (−60% to −23%) | < 0.01 | 4.43 (4.61) | 2.46 (3.28) | −43% (−62% to −24%) | < 0.01 |
| Haematological tests† | 4 (3) | 2 (3) | −37% (−57% to −18%) | < 0.01 | 4.71 (4.43) | 2.98 (3.97) | −37% (−57% to −17%) | < 0.01 |
| Renal and liver function tests | 4 (4) | 2 (3) | −48% (−68% to −29%) | < 0.01 | 7.81 (7.84) | 3.75 (5.93) | −52% (−72% to −32%) | < 0.01 |
| Vitamins | 1 (1) | 1 (1) | −2% (−29% to 24%) | 0.87 | 8.29 (9.68) | 8.13 (9.79) | −2% (−28% to 24%) | 0.88 |
| Endocrinological tests‡ | 1 (0.8) | 1 (0.7) | 4% (−29% to 37%) | 0.80 | 3.54 (5.32) | 3.67 (4.71) | 4% (−28% to 24%) | 0.82 |
| Other laboratory tests§ | 3 (4) | 3 (4) | 11% (−12% to 33%) | 0.35 | 55.14 (134.85) | 63.45 (133.35) | 15% (−40% to 70%) | 0.59 |
| Electrophysiological examinations¶ | 1.8 (0.72) | 2.0 (0.80) | 14% (4% to 24%) | 0.01 | 131.97 (54.93) | 150.09 (59.62) | 14% (4% to 23%) | < 0.01 |
Values are mean (SD).
*Mean relative difference for the number and costs of investigations; †includes full blood count, indices, and erythrocyte sedimentation rate; ‡includes thyroid funtion tests; §includes immunological tests, neuronal antibody tests, serological tests, and cerebrospinal fluid investigations; ¶electromyography, nerve conduction, and other neurophysiological investigations.
CI, confidence interval.
After guideline implementation, the time to reach a diagnosis decreased non‐significantly by two weeks. Fewer patients were referred from the general hospitals to a neuromuscular expertise centre (14% before versus 3% after guideline implementation; p = 0.003).
In one third of patients investigations were performed repeatedly and, because the results were always the same, these were non‐contributive. After guideline implementation, there was a significant reduction in the number and costs of investigations for each patient for electrolytes and glucose, haematological tests, and renal and liver function tests. Few, yet costly, other laboratory investigations were performed for each patient but none of these was contributive. Thus, there was a significant reduction (27%; 95% confidence interval (CI), −44% to −10%; p < 0.01) in the total number of investigations for each patient after guideline implementation, but no reduction in the total costs. Because in the general hospitals electromyographic investigations were more often performed after guideline implementation (in 62% versus 77% of patients; p = 0.01), there was an increase in the number and costs of electrophysiological investigations.
After guideline implementation, the numbers and costs of investigations for each patient were lower in patients (n = 37) in whom the workup was according to the guideline when compared with the other patients (n = 100): the number of investigations was on average reduced by 93% (95% CI, 78% to 107%; p < 0.01) and the costs by 80% (95% CI, 41% to 118%; p < 0.01).
Discussion
The workup of chronic polyneuropathy according to our restrictive guideline seemed to have sufficient diagnostic reliability and was more cost efficient, despite low guideline adherence. The diagnoses for polyneuropathy remained similar and no causes were overlooked. We hypothesise that fewer causes of polyneuropathy are overlooked, because the workup and evaluation of the results of the investigations according to the guideline is more structured.
Guideline adherence was low and extensive investigations were performed in most patients, yet the proportion of patients with idiopathic polyneuropathy was high compared with other studies.2,7,8,9,10,11,13,18 One reason could be that the UMC is a tertiary referral centre where many patients with idiopathic polyneuropathy are seen. Another reason could be the lack of inquiry into drinking habits and family history of polyneuropathy in general hospitals, even though alcoholic and hereditary neuropathy are frequently overlooked causes of chronic polyneuropathy.7,8,10,21
In the general hospitals, agreement on electrophysiological classification increased after guideline implementation because an incorrect diagnosis of demyelinating polyneuropathy was made less often. Nonetheless, electrophysiological investigations in the general hospitals were more valuable in confirming the clinical diagnosis rather than distinguishing accurately between a demyelinating and axonal polyneuropathy. However, the perfect agreement in classification when the electrophysiological guideline criteria are used and the classification in the UMC suggest that proper application of the guideline can distinguish a demyelinating from an axonal polyneuropathy with sufficient reliability. In favour of this is our finding that about one in 10 chronic polyneuropathies is demyelinating, as reported previously.2,18
Diagnostic delay was somewhat shorter after guideline implementation. More frequent application of the guideline may further decrease diagnostic delay, the number and costs of investigations, and the number of consultations. If there is characteristic polyneuropathy with a common cause, a diagnosis might even be established in one consultation, without further investigations. In addition, a strict workup according to the guideline may further reduce repeated and costly investigations, which usually lack additional diagnostic value.
The low guideline adherence in our study was also found by others, and further improvement of cost efficiency depends on effective guideline implementation and adherence, ensured by stringent evaluations and feedback.23 Lack of these could be an explanation for the limited cost efficiency in our study. Although the neurologists had no knowledge of the guideline before the implementation, their conventional workup resembled that of the guideline in 40 of 173 (23%) patients, which could also account for the limited cost efficiency. In addition, there may have been bias through the Hawthorne effect. Because the neurologists knew that their performance was being monitored this may have changed their behaviour, which could have influenced the impact of the guideline. A randomised study, a balanced incomplete block design, or a controlled before and after study would not have circumvented this problem.24
In conclusion, more rigorous evaluation strategies after implementation may increase guideline adherence and consequently improve cost efficiency without loss of diagnostic reliability.
Supplementary Material
Acknowledgements
This study was supported by Programma CVZ/VAZ doelmatigheidsprojecten “Doelmatigheid Academische Ziekenhuizen”, grant number 99165. We are very grateful to the following centres and their neurologists for their participation: Meander Medisch Centrum/Ziekenhuis Eemland Amersfoort (Dr A Hovestadt); Gooi Noord Medisch Centrum/Ziekenhuis Hilversum (Dr JJ Claus); Diakonessenhuis Utrecht (Dr RPM Bruyn); MESOS Medisch Centrum Utrecht/Ziekenhuis Overvecht (Dr RD Verweij; currently Rivierenland Ziekenhuis Tiel); and Diakonessenhuis/Lorentz Ziekenhuis Zeist (Dr RJ Blom).
Abbreviations
CI - confidence interval
UMC - University Medical Centre
Footnotes
Competing interests: none declared
References
- 1.MacDonald B K, Cockerell O C, Sander J W.et al The incidence and lifetime prevalence of neurological disorders in a prospective community‐based study in the UK. Brain 2000123665–676. [DOI] [PubMed] [Google Scholar]
- 2.Mygland Å, Monstad P. Chronic polyneuropathies in Vest‐Agder, Norway. Eur J Neurol 20018157–165. [DOI] [PubMed] [Google Scholar]
- 3.Davis L E, Eisen S A, Murphy F M.et al Clinical and laboratory assessment of distal peripheral nerves in Gulf War veterans and spouses. Neurology 2004631070–1077. [DOI] [PubMed] [Google Scholar]
- 4.The Italian General Practitioner Study Group (IGPSG) Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation in two Italian regions. I. Prevalence and general characteristics of the sample. Neurology 1995451832–1836. [DOI] [PubMed] [Google Scholar]
- 5.The Italian Longitudinal Study on Aging Working Group Prevalence of chronic diseases in older Italians: comparing self‐reported and clinical diagnoses. Int J Epidemiol 199726995–1002. [DOI] [PubMed] [Google Scholar]
- 6.McLeod J G. Investigation of peripheral neuropathy. J Neurol Neurosurg Psychiatry 199558274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyck P J, Oviatt K F, Lambert E H. Intensive evaluation of referred unclassified neuropathies yields improved diagnosis. Ann Neurol 198110222–226. [DOI] [PubMed] [Google Scholar]
- 8.McLeod J G, Tuck R R, Pollard J D.et al Chronic polyneuropathy of undetermined cause. J Neurol Neurosurg Psychiatry 198447530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Notermans N C, Wokke J H J, van der Graaf Y.et al Chronic idiopathic axonal polyneuropathy: a five year follow up. J Neurol Neurosurg Psychiatry 1994571525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubec D, Mullbacher W, Finsterer J.et al Diagnostic work‐up in peripheral neuropathy: an analysis of 171 cases. Postgrad Med J 199975723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jann S, Beretta S, Bramerio M.et al Prospective follow‐up study of chronic polyneuropathy of undetermined cause. Muscle Nerve 2001241197–1201. [DOI] [PubMed] [Google Scholar]
- 12.Hughes R A, Umapathi T, Gray I A.et al A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain 20041271723–1730. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg N R, Vermeulen M. Chronic idiopathic axonal polyneuropathy revisited. J Neurol 20042511128–1132. [DOI] [PubMed] [Google Scholar]
- 14.Bouche P, Maisonobe T, Le Forestier N. Conduite à tenir devant une polyneuropathie. Rev Neurol (Paris) 1998154552–556. [PubMed] [Google Scholar]
- 15.England J D, Asbury A K. Peripheral neuropathy. Lancet 20043632151–2161. [DOI] [PubMed] [Google Scholar]
- 16.Hughes R A. Peripheral neuropathy. BMJ 2002324466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suarez G A, Chalk C H, Russell J W.et al Diagnostic accuracy and certainty from sequential evaluations in peripheral neuropathy. Neurology 2001571118–1120. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg N R, Portegies P, de Visser M.et al Diagnostic investigation of patients with chronic polyneuropathy: evaluation of a clinical guideline. J Neurol Neurosurg Psychiatry 200171205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourmand R. Evaluating patients with suspected peripheral neuropathy: do the right thing, not everything. Muscle Nerve 200226288–290. [DOI] [PubMed] [Google Scholar]
- 20.Johannsen L, Smith T, Havsager A ‐ M.et al Evaluation of patients with symptoms suggestive of chronic polyneuropathy. Journal of Clinical Neuromuscular Disease 2000347–52. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg N R, Slotema C W, Hoogendijk J E.et al Follow up of patients with signs and symptoms of polyneuropathy not confirmed by electrophysiological studies. J Neurol Neurosurg Psychiatry 200576879–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franssen H, Notermans N C, Wieneke G H. The influence of temperature on nerve conduction in patients with chronic axonal polyneuropathy. Clin Neurophysiol 1999110933–940. [DOI] [PubMed] [Google Scholar]
- 23.Grimshaw J M, Russell I T. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet 19933421317–1322. [DOI] [PubMed] [Google Scholar]
- 24.Verstappen W H J M, Van der Weide T, Ter Riet G.et al Block design allowed for control of the Hawthorne effect in a randomized controlled trial of test ordering. J Clin Epidemiol 2004571119–1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.