Abstract
Cellular and humoral immunity have been implicated in the pathogenesis of atherosclerosis. To determine whether an intact immune system is necessary for the formation of atherosclerotic lesions, we have generated immunodeficient mice with hypercholesterolemia and atherosclerosis by crossbreeding the apolipoprotein E (apoE)-deficient mouse with the recombinase activating gene 1 (Rag-1) knockout mouse. Chow-fed immunodeficient mice with targeted disruption in both apoE and Rag-1 (E0/R0) had a 2-fold decrement in aortic root lesion size at 16 weeks of age, compared with immunocompetent littermates, which were heterozygotes at the Rag-1 locus (E0/R1). Nearly all atherosclerotic lesions from chow-fed animals were limited to raised foam cell fatty streaks. In contrast, when a second group of animals was fed a high-fat Western-type diet to accelerate lesion development, there were no differences in either aortic root lesion size or the percent of the total aorta occupied by lesions. Fibrous plaques with well-defined caps and necrotic cores were detected in both Western diet-fed E0/R0 and E0/R1 animals. We conclude that T and B lymphocytes play only a minor role in the rate of forming foam cell lesions, and they are not necessary for the formation of fibroproliferative plaques.
Immunocytochemical staining for cell surface markers has revealed both CD4 and CD8 T lymphocytes associated with macrophages in human atherosclerotic plaques (1–3). B lymphocytes are generally not detected in atherosclerotic lesions (4); however, circulating autoantibodies to epitopes of oxidized lipoproteins have been found in patients with atherosclerosis (5, 6). Despite these important observations, the role of T and B cells in atherogenesis has not been proven. To determine whether T and B lymphocytes are necessary for the formation of atherosclerotic plaques, the recombinase-activating gene 1 (Rag-1) knockout mouse (7) was crossed with a hypercholesterolemic and atherosclerosis-prone mouse, the apolipoprotein E (apoE)-deficient mouse (8). Rag-1-deficient mice have a persistent combined immunodeficiency with immature, dysfunctional B and T lymphocytes, as a result of defective VDJ recombination (7). Therefore, T lymphocytes cannot mature into CD4+ helper or into CD8+ suppressors cells, and B cells are unable to synthesize immunoglobulin. apoE-deficient mice have spontaneous elevations in plasma cholesterol when fed a chow diet and develop fibroproliferative atherosclerotic lesions histologically similar to human atherosclerosis (9). CD4+ and CD8+ T lymphocytes have been detected in atherosclerotic lesions of apoE-deficient mice (10, 11). In addition, high titers of circulating autoantibodies to oxidized lipoproteins are detected in apoE-deficient mouse plasma (12).
In the present report, apoE-deficient mice (E0) were bred with Rag-1-deficient mice (R0), to yield, after several rounds of mating, immunodeficient double knockouts (E0/R0) and immunocompetent E0, Rag-1 heterozygotes (E0/R1). Atherosclerotic lesion size and progression were measured in E0/R0 and E0/R1 mice. There was a small decrement in foam cell lesion size in immunodeficient mice on the chow diet; however, impaired cellular and humoral immunity did not affect fibrous plaque formation or lesion size in mice fed a high-fat diet.
METHODS
Mice.
All mice were housed in a specific pathogen-free environment. The creation of the apoE-deficient mouse used in this study has been described previously (8). C57BL/6 × 129 apoE-deficient female mice were bred to Rag-1-deficient male mice (The Jackson Laboratory) on a C57BL/6 × 129 background. Heterozygous F1 progeny were interbred, yielding 9 possible F2 genotypes. apoE-deficient, Rag-1 heterozygotes (E0/R1) and double knockout (E0/R0) were intercrossed to yield F3 progeny, which served as subjects in this experiment. Since mice heterozygous for Rag-1 are immunocompetent and indistinguishable from wild-type mice (7), Rag-1 heterozygotes (R1) were used in place of mice homozygous for the wild-type allele. This enabled all mice of this cross to be used. Comparisons were made between littermates to minimize background strain differences. Screening for apoE or Rag-1 deficiency was done by phenotypic assays. Blood was obtained from the tail vein, and apoE deficiency in these mice was detected by elevation of serum cholesterol as described below. Homozygous Rag-1 deficiency phenotype was detected by the absence of serum IgM by a dot-blot assay (see below).
Quantitative Atherosclerosis Measurements.
All progeny of each E0/R1 × E0/R0 intercross were weaned at 3 weeks and either fed a standard chow diet [PicoLab Rodent 20 (5053): 20% protein from plant and animals sources, 4.5% (wt/wt) fat, 0.02% (wt/wt) cholesterol, no casein, no sodium cholate] or a Western-type diet [Teklad Adjusted Calories 88137, 21% (wt/wt) fat, 0.15% (wt/wt) cholesterol, 19.5% (wt/wt) casein, no sodium cholate]. At 16 weeks of age, mice were anesthetized and blood was collected by means of left ventricular puncture into syringes containing EDTA. The circulatory system was perfused with 0.9% NaCl by cardiac intraventricular canalization. The heart and ascending aorta, including the aortic arch, were removed, and the heart, containing the aortic root, was fixed in phosphate-buffered formalin and processed for the aortic root quantitative atherosclerosis assay as previously described (13). The unfixed aortic arch was frozen in OCT embedding medium using liquid nitrogen-cooled isopentane. OCT blocks were stored at −70°C until sectioning for immunocytochemistry. Additional animals were sacrificed at 22 weeks on the Western-type diet for surface lesion area determination by an en face method (12). The entire aorta was removed, opened by cutting longitudinally, and stained with oil red O.
Plasma Cholesterol Analysis.
A double equilibrium density centrifugation protocol was used to accomodate the small volumes of plasma obtained from mice. Very low density lipoprotein (VLDL) and chylomicrons (d < 1.006 g/ml) were separated by overlaying 60 μl of PBS onto 60 μl of plasma, followed by centrifugation for 3 hr in a Beckman Airfuge. The lower 60 μl d > 1.006 g/ml fraction was withdrawn with a Hamilton syringe and adjusted to 1.063 g/ml by the addition of 60 μl of a potassium bromide solution at 1.12 g/ml and was spun overnight. The 60-μl bottom fraction was removed and assigned as the high density lipoprotein (HDL) cholesterol fraction. Total plasma cholesterol and HDL cholesterol were determined enzymatically (Boehringer Mannheim). Non-HDL cholesterol was calculated as the difference between total and HDL cholesterol.
Detection of Plasma Immunoglobulins.
Plasma from mice, including plasma samples from control and Rag-1-deficient mice, originally obtained from The Jackson Laboratory, were spotted onto nitrocellulose membranes. After a 30-min incubation with a casein blocker (Pierce), membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse IgM (Pierce; 1:20,000 dilution in casein blocker) or conjugated IgG (1:5,000 dilution in casein blocker) for 1 hr at room temperature. Membranes were washed with PBS/Tween 20 (0.05%), incubated with ECL reagent (Amersham) for 1 min, wrapped in cellophane, and exposed to x-ray film. An exposed spot on film indicated the presence of IgM or IgG and the presence of the Rag-1 gene.
Immunocytochemistry.
Aortic arch 6-μm frozen sections were cut and allowed to adhere to Fisher Plus slides. Slides were stained with oil red O and/or hematoxylin and light green or processed for immunocytochemistry as follows. Sections were fixed with acetone for 5 min and were subjected to a 0.3% hydrogen peroxide incubation to block endogenous peroxidase activity, followed by blocking in normal rabbit serum. Antibodies were applied to detect the presence of T lymphocytes (CD4, PharMingen, 1:100), macrophages (MOMA-2, Serotec, 1:100), and smooth muscle cells (alkaline phosphatase-conjugated α-actin, Sigma Immunochemicals, 1:60). Primary antibody was applied to the sections for 90 min at room temperature. For CD4 and MOMA-2 staining, after washing, a biotinylated secondary antibody was applied for 45 min, followed by a wash and a 30-min incubation with avidin–biotin complex linked to horseradish peroxidase (Vector-Elite, Vector Laboratories). Peroxidase was detected with 3-amino-9-ethylcarbazole (Dako). For the detection of smooth muscle cells, alkaline phosphatase substrate (Vector Red) was added after primary antibody incubation. Controls were performed with either an irrelevant isotype-matched monoclonal antibody or in the absence of primary antibody, and no background staining was visualized.
Statistics.
Comparisons between groups was performed using a two-way ANOVA with the Newman–Keuls post-hoc test. Since atherosclerotic root lesion area was not normally distributed, the Mann–Whitney U test was used to evaluate differences in aortic root lesion area. Multivariate linear regression was performed to evaluate the effect of several variables on lesion area. Statistica 5.0 for Windows (Statsoft) was used for all statistical calculations.
RESULTS
This study was designed to evaluate atherosclerotic lesion size and progression in immunocompetent (E0/R1) and immunodeficient (E0/R0) littermates. Entire litters from E0/R1 × E0/R0 intercrosses were weaned onto either chow or the Western-type diet and sacrificed at 16 weeks of age. Immunodeficient animals (E0/R0) were identified by the complete absence of plasma IgM using the dot-blot assay (data not shown, see Methods). E0/R0 mice also lacked IgG (data not shown). Immunodeficient animals appeared healthy, and body weights were not significantly different from immunocompetent littermates (Table 1). Males weighed significantly more than females within each genotype as expected. Chow-fed E0/R1 males but not females had 30% higher total and non-HDL cholesterol but similar HDL cholesterol compared with E0/R0 littermates (Table 1). Within the E0/R1 genotype, chow-fed males had higher total and non-HDL cholesterols than chow-fed females (Table 1).
Table 1.
Body weights and plasma cholesterol levels of immunocompetent and immunodeficient mice
| Diet | Genotype | Gender | Weight, g | TC, mg/dl | HDL C, mg/dl | Non-HDL C, mg/dl |
|---|---|---|---|---|---|---|
| Chow | E0/R1 | Male (n = 11) | 28 ± 1 | 559 ± 53 | 28 ± 4 | 531 ± 50 |
| Female (n = 9) | 23 ± 1 | 344 ± 33 | 25 ± 3 | 320 ± 30 | ||
| Combined | 25 ± 1 | 467 ± 40 | 27 ± 3 | 441 ± 38 | ||
| E0/R0 | Male (n = 9) | 25 ± 1 | 419 ± 36* | 27 ± 2 | 393 ± 35† | |
| Female (n = 8) | 21 ± 1 | 357 ± 63 | 28 ± 4 | 329 ± 62 | ||
| Combined | 23 ± 1 | 388 ± 36 | 28 ± 2 | 361 ± 35 | ||
| Western | E0/R1 | Male (n = 7) | 33 ± 2 | 1128 ± 115 | 28 ± 5 | 1100 ± 14 |
| Female (n = 6) | 26 ± 2 | 1228 ± 206 | 32 ± 7 | 1196 ± 205 | ||
| Combined | 29 ± 2 | 1183 ± 119 | 30 ± 4 | 1153 ± 119 | ||
| E0/R0 | Male (n = 7) | 33 ± 2 | 887 ± 192 | 32 ± 3 | 855 ± 191 | |
| Female (n = 6) | 29 ± 1 | 1108 ± 204 | 25 ± 3 | 1082 ± 204 | ||
| Combined | 31 ± 1 | 989 ± 138 | 29 ± 2 | 960 ± 137 |
TC, total cholesterol; HDL-C, HDL cholesterol. Values are means ± SEM. ∗, P = 0.055; †, P = 0.046, compared with chow-fed E0/R1.
Atherosclerotic lesion area was evaluated in three separate assays: in the aortic root on the chow diet, in the aortic root on the Western diet, and in the entire aorta on the Western diet in an en face preparation. Aortic root lesion area in chow-fed E0/R0 male mice was approximately half of that in E0/R1 chow-fed male mice (Table 2). Although lesion size was also lower in chow-fed female E0/R0 mice than in E0/R1 females, the difference was not statistically significant. When chow-fed males and females were combined, there was a 42% decrease in lesion size in the E0/R0 mice (Table 2). Examination of aortic root lesions on the chow diet revealed that nearly all lesions were foam cell lesions (data not shown). To determine if the differences in plasma cholesterol in chow-fed mice could account for the differences in lesion area, multivariate linear regression was performed, using data from chow-fed mice. Rag-1 genotype accounted for 25% (r = 0.508; P = 0.002) of the variance in lesion area in the model. HDL cholesterol was negatively correlated with lesion area (r = −0.491; P = 0.003), but there were no differences in mean HDL cholesterol between groups. Total cholesterol, non-HDL cholesterol, gender, and weight did not account for aortic root lesion area in this model. Therefore, Rag-1 deficiency accounts for a significant proportion of the decrease in lesion size in E0/R0 mice.
Table 2.
Atherosclerotic lesion areas of immunocompetent and immunodeficient mice
| Diet | Genotype | Gender | Lesion area, μm2 |
|---|---|---|---|
| Chow | E0/R1 | Male (n = 12) | 136,144 ± 27,365 (132,400) |
| Female (n = 9) | 145,117 ± 26,466 (161,100) | ||
| Combined | 139,990 ± 18,879 (139,900) | ||
| E0/R0 | Male (n = 9) | 70,609 ± 7745 (74,120)* | |
| Female (n = 9) | 91,262 ± 14,840 (74,870) | ||
| Combined | 80,936 ± 8,497 (74,490)† | ||
| Western | E0/R1 | Male (n = 4) | 433,767 ± 65,972 (432,200) |
| Female (n = 6) | 545,441 ± 46,603 (520,500) | ||
| Combined | 500,771 ± 40,458 (497,700) | ||
| E0/R0 | Male (n = 7) | 462,361 ± 49,368 (469,400) | |
| Female (n = 6) | 413,229 ± 53,833 (405,300) | ||
| Combined | 439,685 ± 35,545 (448,800) |
Values are expressed as mean lesion area ± SEM. Median lesion areas are in parentheses. ∗, P = 0.047 by Mann–Whitney U test as compared with male E0/R1; †, P = 0.022 by Mann–Whitney U test as compared with combined male and female E0/R1.
Several litters were fed the Western diet to accelerate atherogenesis (9). On the Western diet, total cholesterol increased compared with the chow diet, but total, non-HDL, and HDL cholesterol were not significantly different between genotypes (Table 1). There was no significant difference in aortic root lesion size in E0/R0 mice compared with E0/R1 mice of similar sex (Table 2). The extent of the thoracic and abdominal aorta occupied by lesions was also measured in 22-week-old Western diet-fed female littermates by using an en face assay (12). There was no significant difference (by unpaired t test) in aortic lesion surface area, as E0/R1 mice had lesions covering 12% ± 2% (n = 5) of the aortic surface, and E0/R1 mice had lesions covering 11% ± 1% (n = 3) of the aortic surface.
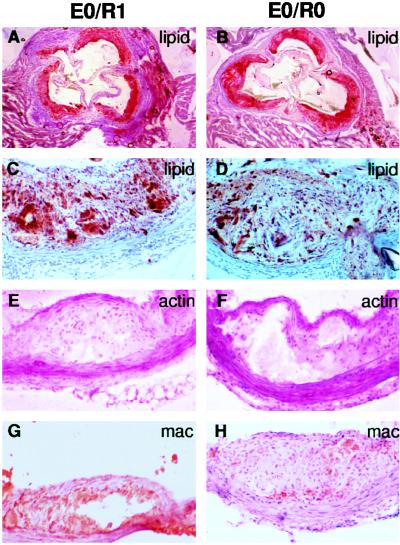
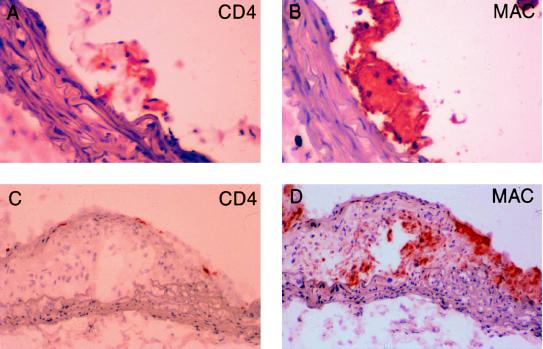
To determine if immunodeficiency had an effect on lesion progression and plaque morphology, aortic root and aortic arch lesions were evaluated for the presence of fibrous caps and necrotic cores. Aortic root lesions from Western diet-fed E0/R0 mice were similar in size (Fig. 1B) to lesions from immunocompetent E0/R1 mice (Fig. 1A). Aortic root lesions from Western diet-fed E0/R0 had well-formed fibrous caps and necrotic cores (Fig. 1D) and were indistinguishable from lesions found in E0/R1 mice (Fig. 1C). Immunostaining using antibodies against smooth muscle cells (Fig. 1 E and F) and macrophages (Fig. 1 G and H) also revealed the presence of well-formed fibrous caps and necrotic cores in aortic arch lesions from both E0/R0 and E0/R1 mice. Antibody staining for CD4+ T lymphocytes revealed T lymphocytes in fatty streaks (Fig. 2A) and fibrous plaques (Fig. 2C) in the aortic arch of immunocompetent E0/R1 animals and their absence in E0/R0 mice (data not shown). Immunostaining of serial sections of the aortic arch reveals macrophage foam cells adjacent to CD4+ T lymphocytes in early foam cell lesions (Fig. 2 A and B). In fibrous lesions, CD4+ T lymphocytes are less prevalent (Fig. 2C) than in fatty streaks (Fig. 2A) and are principally located in the subendothelium and not adjacent to clusters of foam cells (Fig. 2D).
Figure 1.
Aortic root and arch lesions from 16-week-old Western diet-fed mice. (Left) apoE-deficient, Rag-1 heterozygote mouse lesions (E0/R1). (Right) apoE-deficient, Rag-1-deficient mouse lesions (E0/R0). (A and B) Oil red O staining for lipid in aortic root lesions (4× objective). (C and D) Oil red O staining for lipid in aortic root lesions (20× objective) reveals fibroproliferative lesions with fibrous caps and cholesterol clefts. (E and F) α-Actin antibody staining (dark magenta) in aortic arch fibroproliferative lesions demonstrates smooth muscle cells in the fibrous cap (20× objective). (G and H) Macrophage antibody staining (red-brown) in aortic arch lesions with necrotic cores (20× objective).
Figure 2.
Aortic arch lesions from 16-week-old Western diet-fed apoE-deficient, Rag-1 heterozygote mice. (A) Foam cell lesion stained for CD4+ T lymphocytes (red; 40× objective). (B) Macrophage antibody staining (red) of the same lesion as in A. (C) Fibrous lesion stained with CD4+ antibody demonstrates subendothelial T lymphocytes in a fibrous plaque (20× objective). (D) Macrophage antibody staining of the same lesion as in C demonstrates patchy macrophage staining and the presence of a necrotic core.
DISCUSSION
There is an abundance of circumstantial evidence that T and B cells play a role in atherogenesis. T lymphocytes are found in human atherosclerotic plaques and therefore T cells have been implicated in the pathogenesis of atherosclerosis (1–3). The possibility of B cell involvement in atherogenesis has been based on the finding of immunoglobulin in atherosclerotic lesions (14) and circulating autoantibodies to oxidized low density lipoprotein (LDL) in both humans (5, 6) and animals (12, 15) with atherosclerosis. Several studies also suggest that T lymphocytes in lesions are in the activated state and influence gene expression in neighboring cells. CD25, a marker of T cell activation, colocalized with CD4+ T cells in human (1) and apoE-deficient mouse (11) lesions. PCR amplification of interferon γ mRNA (16) and immunodetection of interferon γ protein in atherosclerotic lesions (17) suggest that T cells release cytokines which may influence gene expression in neighboring cells of the lesion. A population of smooth muscle cells, endothelial cells, and macrophages in human lesions express class II histocompatibility antigens, and this expression has been proposed to be due to interactions with activated T lymphocytes (1). A study by Hansson et al. (18) suggests that T cell activation may result from interactions with macrophages and oxidized lipoproteins. CD4+ T cells clonally derived from human atherosclerotic lesions were cultured with native or oxidized LDL in the presence of autologous monocytes. Several of these T cell lines proliferated and secreted cytokines only upon incubation with oxidized LDL, thereby suggesting that the original plaque-derived T cells had been primed against oxidized LDL in vivo. This response was dependent upon HLA class II-restricted macrophage antigen presentation because HLA-DR monoclonal antibodies blocked the T cell response (18).
Despite these important observations, the relative importance of T and B cells in atherosclerotic lesion formation and progression has not been evaluated. The present study addresses the question whether the absence of functional T and B lymphocytes will affect atherosclerosis in induced mutant mice which spontaneously develop atherosclerosis. apoE-deficient mice were bred with Rag-1-deficient mice, a nonleaky model of severe immunodeficiency, to finally yield immunocompetent E0/R1 and immunodeficient E0/R0 littermates. Although foam cell lesion size was nearly half the size in chow-fed E0/R0 mice, there was no difference in lesion size and progression to fibrous lesions, or in the surface area covered by lesions, in Western diet-fed animals. Thus, functional T and B lymphocytes were not necessary for the formation of fibroproliferative lesions in immunodeficient E0/R0 animals, as advanced lesions of E0/R0 mice had well-defined fibrous caps and necrotic cores which were morphologically identical to the lesions of E0/R1 mice. Smooth muscle proliferation, synthesis of a fibrous cap, and the formation of a necrotic core can apparently occur without the influence of T lymphocytes or immunoglobulins. Since plaque rupture and thrombosis have not been noted in lesions in the apoE-deficient mouse, the role of lymphocytes in plaque destabilization and plaque rupture cannot be addressed in this study.
CD4+ T cells were present in both foam cell and fibroproliferative lesions of Western diet-fed E0/R1 mice. Hansson and co-workers (11) have reported relatively abundant CD4+ T cells in aortic lesions from apoE-deficient mice fed a high cholesterol diet containing cholic acid, and fewer T lymphocytes in chow-fed apoE-deficient mice. In contrast, Daugherty and co-workers (10) reported an extremely low number of T lymphocytes present in lesions from apoE-deficient mice fed a high-fat, high-cholesterol, cholic acid-containing diet, although T lymphocytes were more abundant in early lesions. In the present study, CD4+ T lymphocytes were present in appreciable numbers throughout foam cell lesions from Western diet-fed E0/R1 animals, whereas fibrous lesions from E0/R1 mice had fewer lymphocytes distributed subendothelially. Thus the frequency of T lymphocytes present in atherosclerotic lesions in apoE-deficient mice appears to depend upon the lesion stage. Other factors such as genetic background and antibodies used to detect lymphocytes may also influence the number of T cells reported. CD4+ T cells were not present in foam cell or fibroproliferative lesions from E0/R0 mice. Despite the absence of lymphocytes in these lesions, they progressed to form fibrous plaques.
Autoantibodies to oxidized lipoproteins have been detected in humans and in animals with hypercholesterolemia. Correlation of antibody titer with atherosclerosis has not been consistently observed (19). Autoantibodies to oxidized lipoproteins may be pathogenic or protective, or may simply reflect the presence of oxidized lipoprotein. A study in rabbits has suggested that the humoral immune system protects against atherosclerotic plaque formation. Immunization of LDL receptor-deficient rabbits with oxidized LDL resulted in increases in the titer of antibodies against oxidized LDL and also reduced atherosclerosis by 30% (20). However, the E0/R0 animals in the current study, which have no circulating IgM or IgG, go on to form fibroproliferative lesions, suggesting that autoantibodies to oxidized LDL may reflect lipoprotein oxidation and are not especially protective against or predisposing toward plaque development.
A previous study to evaluate the role of the cellular immune system in atherogenesis has utilized the C57BL/6 mouse model of atherosclerosis in which mice are fed a high-fat diet containing high levels of cholesterol and cholic acid. Class I-deficient C57BL/6 mice fed an atherogenic diet had a 3-fold increase in foam cell lesion size compared with C57BL/6 controls (21). There was no effect on lesion size in class II-deficient C57BL/6 mice fed the same diet (21). One problem with using this C57BL/6 model is that the diet is inflammatory as shown by its induction of hepatic NF-κB (22). This confounds the study of immune and inflammatory mediators in atherosclerosis. Furthermore, atherosclerosis in this model is observed as mainly immature, nonprogressive, foam cell lesions limited to the aortic root. In a study of cholesterol-fed rabbits, inhibition of cellular immunity by cyclosporine treatment resulted in a 25% increase in atherosclerosis lesion area in the aortic arch (23). Combined, these results suggest that cellular immunity may actually play a protective role against atherosclerosis. On the other hand, the results of the present study suggest that T lymphocytes may have a minor proatherogenic effect on early foam cell lesion formation. It is possible that T lymphocytes interact with macrophages during early fatty streak formation and modulate macrophage recruitment, proliferation, or gene expression, resulting in larger lesions. Alternatively, T cells, which are more prevalent in foam cell than fibrous lesions, may simply add to the lesion size.
The apoE-deficient mouse is an extremely useful model to study the biology of atherosclerosis. Atherosclerosis in the apoE-deficient mice recapitulates human atherosclerosis in a variety of ways. Lesion morphology (8, 9), lesion progression (8, 9), cell types (9–11), diet-responsiveness, and response to elevated HDL (13) are strikingly similar in apoE-deficient mouse atherosclerosis and human atherosclerosis. Since numerous laboratories are presently using the apoE-deficient mouse as a model of human atherosclerosis, defining the role of the lymphocyte in atherosclerotic plaque formation in this mouse model is of great interest. The present study confirms the presence of T lymphocytes in atherosclerotic plaques of the apoE-deficient mouse, but demonstrates that functional T and B cells are not necessary for the formation of atheroma, and that they play a relatively minor role in atherosclerosis in the apoE-deficient mouse. Future studies utilizing knockout mice with selective defects in either T or B cell function would help distinguish the relative contribution of cellular versus humoral immunity on atherosclerosis in the apoE-deficient mouse. In contrast to the present study, the importance of the monocyte–macrophage in atherosclerosis was clearly demonstrated in an experiment involving the apoE-deficient mouse (E0) and the osteopetrotic mouse (24). Osteopetrotic mice (op) have a naturally occurring mutation in macrophage colony-stimulating factor 1 (M-CSF) and decreased number of circulating monocytes (25). Compound mutant mice (E0/op) had less than half as many monocytes and one-quarter to one-tenth as much atherosclerosis, despite a 2.5-fold increase in plasma cholesterol, compared with control E0 littermates (24). Therefore, monocytes may play a larger role than T and B cells during atherogenesis in the apoE-deficient mouse.
Acknowledgments
This research was supported by an Established Investigatorship from the American Heart Association to J.D.S. and by Grant PO1 HL54591 from the National Institutes of Health. H.M.D. is a recipient of an Institutional National Research Service Award.
ABBREVIATIONS
- apoE
apolipoprotein E
- E0
apoE-deficient genotype
- R0
Rag-1-deficient genotype
- R1
Rag-1 heterozygous genotype
- HDL
high density lipoprotein
- LDL
low density lipoprotein
References
- 1.Stemme S, Holm J, Hansson G K. Arterioscler Thromb. 1992;12:206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 2.Hansson G K, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Atherosclerosis. 1988;72:135–141. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- 3.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson G K. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Hansson G, Libby P. In: Atherosclerosis and Coronary Artery Disease. Fuster V, Ross R, Topol E, editors. Philadelphia: Lippincott–Raven; 1996. pp. 557–568. [Google Scholar]
- 5.Salonen J T, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum J L. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 6.Yla-Herttuala S, Palinski W, Rosenfeld M E, Parthasarathy S, Carew T E, Butler S, Witztum J L, Steinberg D. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mombaerts P, Iacomini J, Johnson R, Herrup K, Tonegawa S, Papaioannou V. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 8.Plump A S, Smith J D, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J G, Rubin E M, Breslow J L. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 9.Nakashima Y, Plump A S, Raines E W, Breslow J L, Ross R. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 10.Roselaar S E, Kakkanathu P X, Daugherty A. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Stemme S, Hansson G K. Am J Pathol. 1996;149:359–366. [PMC free article] [PubMed] [Google Scholar]
- 12.Palinski W, Ord V A, Plump A S, Breslow J L, Steinberg D, Witztum J L. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 13.Plump A S, Scott C J, Breslow J L. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yla-Herttuala S, Palinski W, Butler S W, Picard S, Steinberg D, Witztum J L. Arterioscler Thromb. 1994;14:32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Palinski W, Tangirala R K, Miller E, Young S G, Witztum J L. Arterioscler Thromb Vasc Biol. 1995;15:1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y J, Holm J, Nygren S, Bruzelius M, Stemme S, Hansson G K. Arterioscler Thromb Vasc Biol. 1995;15:1995–2002. doi: 10.1161/01.atv.15.11.1995. [DOI] [PubMed] [Google Scholar]
- 17.Hansson G K, Holm J, Jonasson L. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 18.Stemme S, Faber B, Holm J, Wiklund O, Witztum J L, Hansson G K. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ameli S, Hultgardh-Nilsson A, Regnstrom J, Calara F, Yano J, Cercek B, Shah P K, Nilsson J. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 20.Palinski W, Miller E, Witztum J L. Proc Natl Acad Sci USA. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fyfe A I, Qiao J H, Lusis A J. J Clin Invest. 1994;94:2516–2520. doi: 10.1172/JCI117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao F, Andalibi A, deBeer F C, Fogelman A M, Lusis A J. J Clin Invest. 1993;91:2572–2579. doi: 10.1172/JCI116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roselaar S E, Schonfeld G, Daugherty A. J Clin Invest. 1995;96:1389–1394. doi: 10.1172/JCI118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith J, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiktor-Jedrzejczak W W, Ahmed A, Szczylik C, Skelly R R. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]