Abstract
Current guidelines on thrombolysis post stroke with recombinant tissue plasminogen activator (rt‐PA) exclude its use where time of onset is unknown, thus denying some patients potentially beneficial treatment. Contrast enhanced perfusion computed tomography (pCT) imaging can be used together with plain CT and information on clinical deficits to decide whether or not thrombolysis should be initiated even though the exact time of stroke onset is unknown. Based on the results of pCT and CT, rt‐PA was administered to two patients with unknown time of stroke onset; one of the patients also underwent suction thrombectomy. Results in both cases were excellent.
Keywords: ischaemic stroke, penumbra, perfusion CT, thrombolysis
Current guidelines on thrombolysis post stroke with recombinant tissue plasminogen activator (rt‐PA) exclude its use where time of onset is unknown. Up to 25% of all strokes are noted on waking so their time of onset is unclear.1 Other strokes are unwitnessed so their time of onset also cannot be determined (for example, if there is global aphasia). These patients are therefore excluded from potentially beneficial treatment with thrombolysis. Plain computed tomography (CT) in the first few hours after ischaemic stroke is often normal (excluding haemorrhage) or shows only subtle abnormalities. Methods of imaging potentially salvageable brain are being developed. Magnetic resonance imaging (MRI) criteria are used in a few stroke centres for deciding when to initiate thrombolytic therapy, rather than relying on a specific time window.2 Thrombolysis trials based on perfusion/diffusion weighted MRI (DWI/PWI) mismatch are being reported,3 but this technique is not readily available in most hospitals. Contrast enhanced perfusion CT (pCT) imaging is more widely available, and adds only a few minutes to the initial scan protocol. It has limitations compared to DWI/PWI in that it is often restricted to two to four slices, which could miss relevant branch disease,4 and involves iodinated contrast material. However, the two techniques provide comparable information.5,6 We report two cases where the combined information from clinical deficit, plain CT, and pCT was used to make the thrombolysis decision despite unclear time of onset.
Case 1
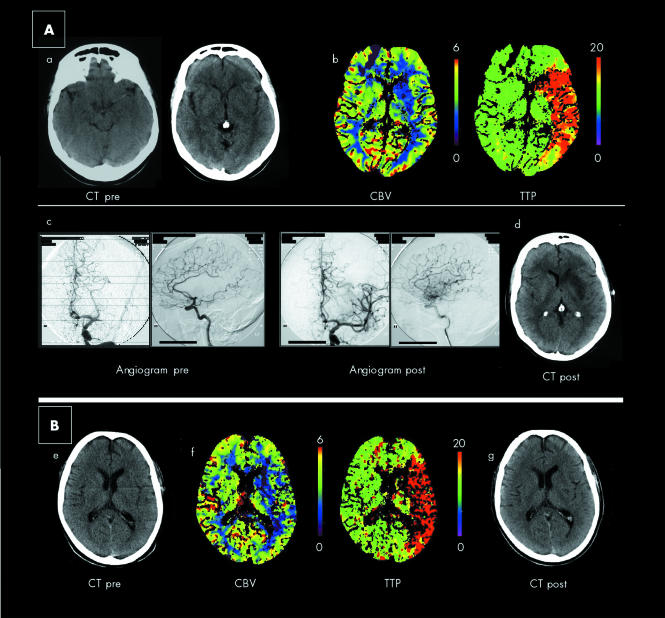
A 58 year old woman was heard falling down beside her bed at 0740 h by her husband. He awoke to find her lying on the floor, not moving her right side, and incoherent. She arrived in hospital at 0900 h with global aphasia, right homonymous hemianopia, dense right hemiplegia, and a National Institute of Health Stroke Scale (NIHSS) score of 18. CT scan at 0930 h (fig 1A) showed a hyperdense left MCA sign and subtle left basal ganglia obscuration. pCT was then performed (Siemens Sensation 4, Berlin, Germany) using methods detailed elsewhere.7,8 Two slices (10 mm thick) were acquired at the level of the basal ganglia and corona radiata. Figure 1B shows the cerebral blood volume (CBV) and time to peak (TTP) maps at the level of the basal ganglia. This shows markedly decreased CBV over the left striato‐capsular area, suggesting irreversible damage, and markedly delayed TTP over the whole left MCA territory, suggesting at risk tissue.4,5,6 This area of irreversible damage indicated that we were probably beyond the 3 h time window, but the large CBV‐TTP mismatch suggested that we might still be within 8 h. Intra‐arterial (IA) thrombolysis has been shown to be of benefit up to 6 h9 and mechanical thrombectomy, often implemented in combination with IA rt‐PA, can be effective up to 8 h,10 and so a decision was made to proceed to cerebral angiography and possible IA therapy.
Figure 1 Plain CT and perfusion CT images of two patients with acute stroke with cerebral angiography images in case 1. (A) Case 1. (a) Plain CT showing hyperdense left MCA and subtle hypodensity of the left basal ganglia. (b) Perfusion CT at the level of the basal ganglia showing CBV (ml/100 g) and TTP (s) maps. This shows markedly decreased CBV over the left striato‐capsular area, and markedly delayed TTP over the whole left MCA territory. (c) Cerebral angiogram showing an occluded left MCA stem (pre) and restored flow to left MCA after thrombolysis, mechanical agitation, and suction thrombectomy (post). (d) Follow up CT showing left basal ganglia infarct. (B) Case 2. (e) Plain CT showing subtle hypodensity in the region of the left head of the caudate, but remaining appearances are normal. (f) Perfusion CT at the level of the basal ganglia. This shows markedly reduced CBV in the left subinsular/caudate regions, while the TTP map shows markedly prolonged circulation to the left MCA territory. (g) Follow up CT showing small left subinsular region infarct. In both cases, final infarct volume on CT correlates with perfusion CT‐CBV map lesion size.
The angiogram showed an occluded left MCA stem (fig 1A, c, pre). A catheter was advanced into the origin and rt‐PA was infused for 40 min. Repeat angiogram showed only minimal recanalisation. Suction thrombectomy was then performed which restored flow at 1440 h (fig 1A, c, post). Following this the speech and power of the patient improved. She was able to give a retrospective history of waking at 0740 h and standing beside her bed before collapsing. Hence, the final time to complete reperfusion was 7 h post stroke. A repeat CT scan on day 2 showed only a striato‐capsular infarct (fig 1A, d), which correlated with the CBV lesion on pCT. The patient was discharged home on day 12 with only mild dysarthria but normal language and no limb weakness.
Case 2
A 73 year old man was under investigation by cardiology for “dizzy spells”. At 0930 h he was found outside the casualty department, slumped against a lamppost, with global aphasia, right homonymous hemianopia, and flaccid right hemiparesis. The NIHSS score was 20. He had a 24 h electrocardiogram monitor on, due to be removed at 1030 h, as confirmed by a letter in his pocket. He also had his car keys, so was assumed to have driven to hospital that morning. Plain CT revealed subtle early ischaemic changes in the left head of the caudate region (fig 1B, e). pCT at 1115 h (fig 1B, f) revealed markedly reduced CBV in the left subinsular/caudate regions, while the TTP scan showed markedly prolonged circulation to the left MCA territory, that is, a mismatch suggesting at risk tissue involving the entire MCA territory.
We assumed that the stroke had only just occurred when he was found, that is, less than 3 h before. In view of the findings on plain CT and pCT, and despite the unclear time of onset, a decision was made to proceed with IV rt‐PA, which was started at 1128 h. By 1610 h power and speech had fully recovered. The patient was then able to clearly describe driving to hospital and parking his car at 0915 h, getting out of the car, and walking towards the hospital. The next thing he remembered was being in a hospital bed. The thrombolysis had been given 2 h 15 min post collapse. Repeat CT on day 2 showed only an infarct in the left subinsular region correlating with the lesion on the CBV map (fig 1B, g). Transcranial Doppler confirmed flow through the left MCA. He was discharged on day 5 having made a full recovery.
Discussion
All current licenses and trials of thrombolysis for stroke rely on having a definite known time of onset. Both these patients would have ordinarily been denied thrombolysis/thrombectomy because we did not know exactly when their strokes occurred.
Due caution must be exercised in administering thrombolysis outside protocols as early experience showed increased rates of intracranial haemorrhage.11 Uncertain time of symptom onset is a major cause for exclusion from treatment,12 however because of the potential benefit of early reperfusion on recovery, additional methods of evaluating which patients stand to benefit have been investigated. In our cases, we used the mismatch on pCT to identify the at risk tissue5,13 as an adjunct to the clinical features and plain CT in guiding decision making on thrombolysis.
Where the expertise is available, the addition of pCT to the plain CT and clinical examination can increase the potential number of patients able to receive thrombolysis. Using these techniques, we reperfused two patients successfully who would otherwise have been unable to benefit from thrombolysis.
Acknowledgements
We thank P Simon Jones BSc, MSc and H Szutowicz, HOCR for their help.
Abbreviations
CBV - cerebral blood volume
CT - computed tomography
DWI - diffusion weighted MRI
IA - intra‐arterial
MRI - magnetic resonance imaging
NIHSS - National Institute of Health Stroke Scale
pCT - perfusion CT
PWI - perfusion weighted MRI
rt‐PA - recombinant tissue plasminogen activator
TTP - time to peak
Footnotes
Competing interests: none declared
Patient consent was obtained for the publication of these details
References
- 1.Fink J N, Kumar S, Horkan C.et al The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke 200233988–993. [DOI] [PubMed] [Google Scholar]
- 2.Hjort N, Butcher K, Davis S M.et al Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke 200536388–397. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Albers G, Al‐Rawi Y.et al The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI‐based 9‐hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 20053666–73. [DOI] [PubMed] [Google Scholar]
- 4.Rother J, Jonetz‐Mentzel L, Fiala A.et al Hemodynamic assessment of acute stroke using dynamic single‐slice computed tomographic perfusion imaging. Arch Neurol 2000571161–1166. [DOI] [PubMed] [Google Scholar]
- 5.Wintermark M, Reichhart M, Cuisenaire O.et al Comparison of admission perfusion computed tomography and qualitative diffusion‐ and perfusion‐weighted magnetic resonance imaging in acute stroke patients. Stroke 2002332025–2031. [DOI] [PubMed] [Google Scholar]
- 6.Schramm P, Schellinger P D, Klotz E.et al Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion‐weighted imaging and diffusion‐weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 2004351652–1658. [DOI] [PubMed] [Google Scholar]
- 7.Gillard J H, Antoun N M, Burnet N G.et al Reproducibility of quantitative CT perfusion imaging. Br J Radiol 200174552–555. [DOI] [PubMed] [Google Scholar]
- 8.Gillard J H, Minhas P S, Hayball M P.et al Assessment of quantitative computed tomographic cerebral perfusion imaging with H2(15)O positron emission tomography. Neurol Res 200022457–464. [DOI] [PubMed] [Google Scholar]
- 9.Furlan A, Higashida R, Wechsler L.et al Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial, Prolyse in Acute Cerebral Thromboembolism. JAMA 19992822003–2011. [DOI] [PubMed] [Google Scholar]
- 10.Gobin Y P, Starkman S, Duckwiler G R.et al MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke 2004352848–2854. [DOI] [PubMed] [Google Scholar]
- 11.Katzan I L, Furlan A J, Lloyd L E.et al Use of tissue‐type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA 20002831151–1158. [DOI] [PubMed] [Google Scholar]
- 12.Kleindorfer D, Kissela B, Schneider A.et al Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population‐based study. Stroke 200435e27–e29. [DOI] [PubMed] [Google Scholar]
- 13.Meuli R A. Imaging viable brain tissue with CT scan during acute stroke. Cerebrovasc Dis 200417(suppl 3)28–34. [DOI] [PubMed] [Google Scholar]