Abstract
Objective
To compare the effects of intravenous methylprednisolone (IVMP) in patients with relapsing‐remitting (RR‐MS), secondary progressive (SP‐MS), and primary progressive multiple sclerosis (PP‐MS).
Methods
Clinical and neurophysiological follow up was undertaken in 24 RR‐MS, eight SP‐MS, and nine PP‐MS patients receiving Solu‐Medrol 500 mg/d over five days for exacerbations involving the motor system. Motor evoked potentials (MEPs) were used to measure central motor conduction time (CMCT) and the triple stimulation technique (TST) was applied to assess conduction deficits. The TST allows accurate quantification of the number of conducting central motor neurones, expressed by the TST amplitude ratio.
Results
There was a significant increase in TST amplitude ratio in RR‐MS (p<0.001) and SP‐MS patients (p<0.02) at day 5, paralleling an increase in muscle force. TST amplitude ratio and muscle force remained stable at two months. In PP‐MS, TST amplitude ratio and muscle force did not change. CMCT did not change significantly in any of the three groups.
Conclusions
In RR‐MS and SP‐MS, IVMP is followed by a prompt increase in conducting central motor neurones paralleled by improvement in muscle force, which most probably reflects partial resolution of central conduction block. The lack of similar clinical and neurophysiological changes in PP‐MS corroborates previous clinical reports on limited IVMP efficacy in this patient group and points to pathophysiological differences underlying exacerbations in PP‐MS.
Keywords: multiple sclerosis, steroid treatment, transcranial magnetic stimulation, triple stimulation technique
High dose intravenous methylprednisolone (IVMP) accelerates recovery from acute relapses of multiple sclerosis1,2,3 and is a standard treatment for acute deterioration in this disease.4 Previous clinical studies have suggested that the treatment is less effective for exacerbations in patients with a progressive disease course,3,4,5 but to our knowledge no study so far has directly compared the IVMP effects between patients with relapsing‐remitting (RR‐MS), secondary progressive (SP‐MS), and primary progressive multiple sclerosis (PP‐MS). IVMP pulse therapy is generally accepted as a safe treatment, without major adverse effects.6 However, negative effects of methylprednisolone on neuronal survival have recently been shown in an animal model of progressive multiple sclerosis,7 emphasising the need for a re‐evaluation of the current therapy regimen.
Impaired motor performance is a major cause of disability in multiple sclerosis, and therefore transcranial magnetic stimulation (TMS) has been used to evaluate treatment effects. The central motor conduction time (CMCT) is easily obtained by TMS,8,9 but changes in CMCT do not generally correlate with improved clinical motor deficit after IVMP treatment in multiple sclerosis,10,11,12 as slowing of conduction is not, or is only marginally, related to clinical function.9,13,14,15
Motor function relates to the number of conducting central motor neurones, which in theory should be reflected by the size of the TMS response. In practice, however, motor evoked potential (MEP) size indices (amplitudes and areas) are not sensitive enough to quantify conduction deficits. Two factors obscure the relation between MEP size and the number of conducting central motor neurones: desynchronisation of the TMS‐induced motor neurone discharges, causing variable degrees of phase cancellation; and repetitive discharges of spinal motor neurones in response to TMS.16 Both factors affect MEP size considerably and unpredictably, and vary between subjects and from one stimulus to the next.16,17 The triple stimulation technique (TST) eliminates the effects of discharge desynchronisation and repetitive discharges on TMS responses, such that an accurate quantification of the proportion of conducting central motor neurones is possible.16 Use of the TST increased the sensitivity for detecting a central motor conduction deficit in multiple sclerosis by a factor of 2.86, and the TST response size correlated with the clinical motor deficit in the patients.18 Moreover, the TST allowed the detection of transient small changes in conduction deficits in patients with multiple sclerosis related to changing body temperature (the “Uhthoff phenomenon”), which correlated well with walking velocity.19
In the present study, multiple sclerosis patients receiving intravenous methylprednisolone for an acute exacerbation involving the motor system were followed clinically and neurophysiologically with the TST. All patients were examined just before the start and at the end of five days of IVMP; in some of the patients a second follow up investigation took place two months after the start of IVMP. Our results point to considerable differences in treatment efficacy between patients with different disease courses.
Methods
Patients
The study was approved by the local ethics committee. All patients gave written informed consent.
Multiple sclerosis group
Forty one patients with definite multiple sclerosis20 were enrolled in the study: 24 with RR‐MS, eight with SP‐MS, and nine with PP‐MS. Disease duration was defined as the time that elapsed between the first disease manifestation (determined clinically or by history) and the current investigation. All patients suffered from a relapse with clinical involvement of the corticospinal tract to the lower limbs (that is, hyperreflexia, extensor plantar response, spasticity, or paresis). A relapse was defined as a newly observed neurological deficit without evidence of spontaneous improvement for at least 24 hours. In all cases, the decision about whether or not methylprednisolone treatment should be started was taken by clinical neurologists who were not involved in the study. In the 17 patients with chronic multiple sclerosis, the disease course was not always known at the start of the treatment, and worsening symptoms or new symptoms were suspected, so that a course of treatment was started. In our department, methylprednisolone treatment is often given in ambiguous clinical situations because of the low risk of short term adverse effects. A clinical examination and electrophysiological investigations were carried out just before starting methylprednisolone treatment ( = day 0; Solu‐Medrol 500 mg/d intravenously for five consecutive days, followed by oral prednisone tapering over 10 days), and at the time of the last methylprednisolone infusion ( = day 5). Twenty eight patients were available for a third investigation at (mean (SD)) 67.6 (10.8) days after the study began ( = 2 months).
Isolated optic neuritis group
To assess disease independent effects of IVMP on pyramidal tract function, clinical and electrophysiological investigations were carried out on days 0 and 5, as described above, in four patients presenting with isolated optic neuritis. An extensive diagnostic workup (clinical examination, analysis of cerebrospinal fluid, somatosensory and motor evoked potentials, and brain magnetic resonance imaging (MRI)) revealed no evidence of multiple sclerosis in these patients. They were assigned to the same IVMP treatment regimen as the multiple sclerosis patients.
Clinical assessment
At the beginning of the study, the extended disability scale score (EDSS21) was calculated for all patients in the multiple sclerosis group. Muscle force in the distal lower limbs (that is, extension and flexion of foot and toes) was graded according to the British Medical Research Council scale (MRC grade 1–5; grade 1 reflecting severe paresis and grade 5 full muscle strength), and the presence or absence of pyramidal signs (hyperreflexia, extensor plantar response, or spasticity) was noted. In the multiple sclerosis group, the leg that was most affected clinically was chosen for electrophysiological testing; in patients with isolated optic neuritis one leg was chosen at random. The same examiner reassessed muscle force and pyramidal signs before neurophysiological testing at day 5 and at 2 months.
Electrophysiological methods
Viking Select apparatus (Nicolet, Madison, Wisconsin, USA) was used for the recordings. Bandpass filters were 2–10 kHz. Recordings were taken from the abductor hallucis muscle using silver electrodes (diameter 0.8 cm) in a belly‐tendon montage.
For TMS, a Magstim 200 stimulator (maximum output 2.0 T) was used, with a double cone (110 mm) hand held coil (Magstim Company, Spring Gardens, Whitland, Dyfed, UK). The coil was placed over the vertex in anterior‐posterior current orientation. Small coil displacements were made in all directions until the position yielding the largest response was found. This position was then maintained throughout the examination. Magnetic stimuli were applied while the patient contracted the target muscle slightly. The MEP latency was defined as the shortest latency in six to eight trials. The CMCT was calculated using the following formula23:
CMCT = MEP latency−(F wave latency+CMAPankle latency−1)/2
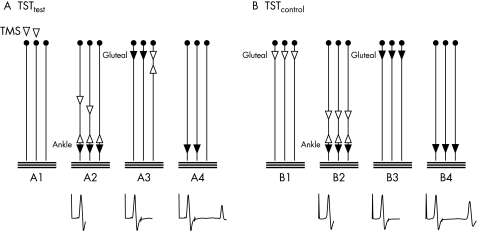
The TST to the lower limbs has been described in detail previously24 (see fig 1 for a summary of the principle). In short, TMS was combined with supramaximal stimuli of the tibial nerve at the ankle and the sciatic nerve at the gluteal fold.24 The peripheral stimuli were given using the two stimulators of the Viking EMG machine. The TST was achieved by using a dedicated software package for the Nicolet Viking apparatus provided by Judex AS (Aalborg, Denmark).
Figure 1 Triple stimulation technique (TST) principle for recordings from lower limbs. The motor tract is simplified to three spinal motor neurones; horizontal lines represent three motor units of the abductor hallucis muscle. Black arrowheads depict action potentials that cause a trace deflection, white arrowheads those that do not. The trace recording is given below at each time point. (A) TSTtest. (A1) A submaximal transcranial stimulus excites two spinal motor neurones of three (white arrowheads). (A2) On two of three neurones, TMS‐induced action potentials descend. Desynchronisation of the two action potentials has occurred (possibly at spinal cell level). After a delay, a maximal stimulus is applied to the tibial nerve at the ankle. This gives rise to a first negative deflection of the recording trace. The antidromic action potentials collide with the descending action potentials on motor neurones 1 and 2. The action potential on neurone 3 continues to ascend. (A3) After a second delay a maximal stimulus is applied to the sciatic nerve at the gluteal fold. On motor neurone 3, the descending action potential collides with the ascending action potential. On neurones 1 and 2, no collision occurs, and action potentials continue to descend on both neurones. During their descent, only a minor degree of desynchronisation occurs, as is typical for peripheral nerves. (A4) Action potentials on motor neurones 1 and 2 evoke a well synchronised muscle response, giving rise to the second negative deflection in the recording trace. Note that motor neurones 1 and 2 were those initially excited by TMS. (B) TSTcontrol. (B1) A maximal stimulus is applied to the sciatic nerve at the gluteal fold. (B2) After a delay, a maximal stimulus applied to the tibial nerve at the ankle is recorded as the first deflection of the TST control trace. (B3) After a delay, a maximal stimulus is applied to the sciatic nerve, evoking action potentials on all neurones. During their descent, a minor degree of peripheral desynchronisation occurs, matching (and calibrating) the desynchronisation that occurred during the TST test procedure. (B4) A well synchronised response from the three motor neurones is recorded as the second deflection of the TST control trace. The test response is quantified as the ratio of TSTtest to TSTcontrol curves.
The delays between the three stimuli were calculated as follows:
Delay I (brain–ankle) = minimum MEP latency−CMAPankle latency
Delay II (gluteal–ankle) = CMAPgluteal latency−CMAPankle latency
The TSTtest curve was then compared to the TSTcontrol curve, obtained by replacing the TMS by a maximal electrical stimulus to the sciatic nerve at the gluteal fold with appropriate delays (delay I = delay II = CMAPgluteal latency − CMAPankle latency).24
Statistics
The TST amplitude was expressed as the amplitude ratio of TSTtest to TSTcontrol (termed the TST amplitude ratio). To test differences between group means, non‐parametric tests were used (the Kruskal–Wallis test for multiple unpaired groups, the Mann–Whitney U test for unpaired two group comparisons, and the Wilcoxon signed rank test for paired two group comparisons). The null hypothesis was rejected at the 0.05 level of significance.
Results
Baseline measurements (day 0)
Clinical and electrophysiological characteristics of the patients are summarised in table 1. The multiple sclerosis groups differed significantly in age (p = 0.005) and disease duration (p = 0.001). Most patients were treated and investigated within two months of the first possible symptom of the current relapse. In some patients, treatment was started later, because of different patterns of referral to our centre (see ranges in table 1). It is noteworthy, however, that non‐parametric testing showed no significant difference in relapse duration before treatment between the patient groups (p = 0.2). At the time of the investigation, only one SP‐MS patient was receiving simultaneous immune modulatory treatment (β interferon).
Table 1 Clinical and electrophysiological characteristics at day 0.
| Disease course | RR‐MS | SP‐MS | PP‐MS | ION | ||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | 24 | 8 | 9 | 4 | ||||
| Sex (female:male) | 11:13 | 5:3 | 5:4 | 1:3 | ||||
| Age (years) | 36 (23 to 54) | 48 (32 to 59) | 54 (32 to 59)* | 46 (43 to 50) | ||||
| Disease duration (months) | 8.5 (0.3 to 240) | 74 (15 to 384) | 12 (12 to 84)* | to | ||||
| Duration of relapse (months) | 1 (0.3 to 7) | 2.4 (0.3 to 4.5) | 4 (0.3 to 7) | 0.8 (0.3 to 1) | ||||
| EDSS | 3 (1.5 to 4) | 4 (2.5 to 6) | 3 (2 to 6.5)* | to | ||||
| Paresis (MRC grade) | 5 (3 to 5) | 4 (3 to 5) | 4 (3 to 5) | 5 (5 to 5) | ||||
| TST amplitude ratio (%) | 68.8 (7.8 to 98.6) | 33.5 (15.9 to 84.4) | 52.6 (3.6 to 86.7) | 95.5 (91.9 to 100) | ||||
| CMCT (ms) | 15.6 (7.2 to 30.4) | 20.8 (17.6 to 28.1) | 21.8 (16.5 to 32.8)* | 12.5 (11.2 to 14.5) |
Values are median (range) or n.
*p<0.01 for comparison of multiple sclerosis patients (Kruskal–Wallis test).
CMCT, central motor conduction time; EDSS, extended disability scale score; ION, isolated optic neuritis; MRC, Medical Research Council; PP‐MS, primary progressive multiple sclerosis; RR‐MS, relapsing‐remitting multiple sclerosis; SP‐MS, secondary progressive multiple sclerosis; TST, triple stimulation technique.
The mean EDSS score was higher for SP‐MS than for RR‐MS patients (p = 0.001), whereas it did not differ significantly between P‐MS and RR‐MS (p = 0.18) and between P‐MS and SP‐MS (p = 0.09). Weakness of the target limb was generally mild to moderate (⩾ grade M4) and did not differ significantly between the multiple sclerosis groups (p = 0.2). Apart from impaired vision, clinical examination was normal in the patients with isolated optic neuritis.
The mean TST amplitude ratio was reduced in all multiple sclerosis groups, but normal in patients with isolated optic neuritis (lower normal limit = 88.4%24). Detailed results are given in table 1. The reduction in the TST amplitude ratio did not differ significantly between the multiple sclerosis groups (p = 0.3). The mean CMCT was slightly prolonged in RR‐MS (upper normal limit = 15.1 ms24), and markedly prolonged in SP‐MS and PP‐MS (p<0.01 compared with RR‐MS). CMCT was within normal limits in all patients with isolated optic neuritis.
Short term follow up (day 0 v day 5)
Methylprednisolone was well tolerated except by one PP‐MS patient who experienced transient arterial hypertension. Muscle force increased significantly in RR‐MS patients (mean force (MRC grade) on day 0 = 4.7 (0.5); on day 5 = 4.9 (0.2); p<0.02). In SP‐MS patients, there was a similar trend (p = 0.07), but no clear change in muscle force could be detected in the PP‐MS group (p = 0.4). In most multiple sclerosis patients, pyramidal signs were less pronounced, but statistical analysis could not be undertaken as quantification of small changes in pyramidal signs is difficult.11 All patients with optic neuritis had normal muscle force and absence of pyramidal signs.
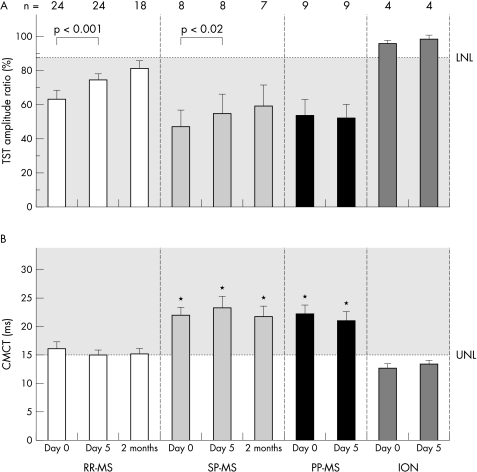
The mean TST amplitude ratio increased significantly in RR‐MS (p = 0.0005) and SP‐MS (p = 0.017), but remained unchanged in PP‐MS and isolated optic neuritis groups (p>0.05; fig 3A; patient examples in fig 2). TST amplitudes increased in most patients with a favourable clinical response to IVMP, but were barely changed in patients who did not respond clinically to the treatment. CMCT remained unchanged in all patient groups (p>0.05; fig 3B).
Figure 3 Mean triple stimulation technique (TST) amplitude ratio (A) and mean central motor conduction time (CMCT) (B) are shown separately for the different patient groups before (day 0) and after intravenous methylprednisolone (IVMP) (day 5 and 2 months). Error bars represent SEM. The dashed line indicates the lower normal limit (LNL = 88.4%) for the TST amplitude ratio and the upper normal limit (UNL = 15.1 ms) for the CMCT, respectively. Significant changes after IVMP are indicated by brackets and the appropriate p value (Wilcoxon signed rank test) on the top of the diagram. For RR‐MS and SP‐MS a significant increase in TST amplitude ratio was found from days 0 to 5. From day 5 to the 2 months follow up, a small but non‐significant increase in TST amplitude ratio occurred in the RR‐MS and SP‐MS groups (the number of available patients is indicated in the headline of the diagram); the number of available PP‐MS patients at 2 months was too small for statistical analysis. There was no significant change in CMCT for any patient group at any time point. Note that the mean CMCT of SP‐MS and PP‐MS was always significantly prolonged compared with RR‐MS (indicated by *). ION, isolated optic neuritis; PP‐MS, primary progressive multiple sclerosis; RR‐MS, relapsing‐remitting multiple sclerosis; SP‐MS, secondary progressive multiple sclerosis.
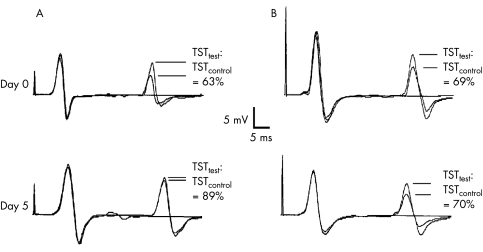
Figure 2 Triple stimulation technique (TST) recordings of a patient with relapsing‐remitting multiple sclerosis (RR‐MS) (A) and with primary progressive multiple sclerosis (PP‐MS) (B), the upper row showing the recordings at day 0, the lower row at day 5. For all recordings, the best TSTtest curve, the TSTcontrol curve, and a baseline curve (obtained by supramaximal stimulation of the tibial nerve at the ankle) were superimposed. The sweep of the traces is delayed and starts with the second stimulus of the TST (electrical stimulation at the ankle). The TST amplitude ratio, calculated by TSTtest:TSTcontrol, is given for each recording. In the RR‐MS patient, there is a clear increase in TST amplitude ratio at day 5 resulting in normalisation of the ratio, whereas it remains reduced and virtually unchanged in the PP‐MS patient. The central motor conduction time (CMCT), obtained by conventional motor evoked potentials (not shown), was normal in RR‐MS (day 0 = 13.9 ms, day 5 = 13.5 ms), but prolonged in PP‐MS (day 0 = 19.2 ms, day 5 = 16.8 ms). Overall the changes in CMCT did not reach significance in any multiple sclerosis group, although there was a clear decrease in CMCT in some patients, as shown here for PP‐MS. Note that the distance between the two negative deflections of the TST recording does not directly reflect CMCT, but depends on the individually calculated delay I and II.
Long term follow up (day 5 v 2 months)
Eighteen RR‐MS patients, seven SP‐MS patients, and three PP‐MS patients without further clinical relapses were available at two months for the third investigation. Five RR‐MS and two SP‐MS patients had begun immunomodulatory treatment in the meantime (six with β interferon, one with glatirameractetate). No significant changes in muscle force or electrophysiological variables where found compared with day 5 (fig 3). The clinical findings remained basically unchanged.
Discussion
We carried out a clinical and neurophysiological follow up in RR‐MS, SP‐MS, and PP‐MS patients receiving IVMP for an acute exacerbation of multiple sclerosis involving the motor system. The principal findings were as follows. First, in RR‐MS and SP‐MS patients, a significant increase in the number of conducting central motor neurones (reflected by an increased TST amplitude ratio) was found after five days of treatment, paralleling an increase of muscle force; at the two months follow up, there was no further significant improvement or deterioration, with a stabilisation of TST amplitude ratio and muscle force. Second, in PP‐MS patients, the treatment caused no significant changes in the TST amplitude ratio or muscle force. Third, CMCT did not change significantly in any of the patient groups.
An untreated control patient group was not included in our study, because we judged it unethical to exclude patients from receiving a treatment considered to be a standard therapeutic approach.4 Nonetheless it is likely that, averaged across patients, the observed changes were related to the IVMP treatment. In all our patients, worsening of symptoms occurred until the start of the treatment, after which progression ceased or symptoms regressed noticeably over the five days of the treatment. The close chronological relation between treatment and amelioration of symptoms suggests that the clinical and electrophysiological changes observed represented an effect of the treatment and not just the natural course of the disease. For an estimation of possible disease‐independent effects of IVMP on central motor conduction, we studied four patients with isolated optic neuritis receiving an IVMP regimen identical to the multiple sclerosis patients. In these patients, whose central motor conduction was unaffected, we observed neither clinical nor neurophysiological changes after IVMP treatment. Likewise, the TST amplitude ratio did not change in the multiple sclerosis patients who did not improve clinically from the IVMP treatment (“non‐responders”).
The increase in TST amplitude ratio observed in our RR‐MS and SP‐MS patients during the IVMP treatment is best explained by a reduction in a central motor conduction block. Conduction block is an important cause of conduction failure and clinical deficit in acute demyelination.15,25 It can result from segmental demyelination (which would not immediately respond to steroid treatment), but may also be caused by oedema or inflammatory cytokines.15,26 IVMP has marked anti‐oedema, anti‐inflammatory, and membrane stabilising properties.4,27 The rapid reduction in conduction deficit in our RR‐MS and SP‐MS patients can thus readily be explained. Theoretically, an increase in the TST amplitude ratio could also be related to an increase in cortical excitability. However, changes in cortical excitability were not found in a previous study of methylprednisolone treatment of acute relapses (at a dose of 1 g/day for five days), using the resting motor threshold as a measure of excitability.10 In the present study, the resting motor threshold was determined only in a small number of patients, where it remained unchanged (results not shown). Overall, it is unlikely that changes in cortical excitability explain our present results.
Several factors may account for the lack of electroclinical improvement in the PP‐MS patient group. First, from histopathological28 and MRI studies,29,30,31 there is increasing evidence that acute inflammation is less prominent in this group of patients, resulting in limited efficacy of IVMP. Second, axonal loss may be more substantial in PP‐MS than in SP‐MS and RR‐MS,32 and conduction deficits caused by axonal loss are not likely to change rapidly in response to IVMP, or to any other treatments. The TST quantifies the number of conducting axons, but a reduction in TST amplitude does not differentiate between loss of axons and conduction block.18 The lack of improvement in follow up investigations is in line with axonal loss having taken place, although persistent conduction block cannot be excluded. Our finding of reduced efficacy of IVMP in PP‐MS corresponds well with previous clinical observations of less frequent and less pronounced IVMP effects in progressive patients.3,5 Nevertheless, the present data do not rule out beneficial effects of IVMP, because further deterioration could have occurred in untreated patients,5 and because the possibility of dose dependent effects in high dose regimens10 were not investigated.
So far, only a few studies have used MEPs for objective assessment of the IVMP effects in patients with multiple sclerosis.10,11,12 None of these studies analysed MEP amplitudes, because the amplitude of conventional MEPs is not sufficient for accurate measurement of conduction deficits. The use of TST circumvents this problem and can demonstrate IVMP associated changes in central motor conduction deficits. Our findings show that there are considerable differences in treatment efficacy between patients in the different clinical groups.
Given the lack of reliable MEP amplitude measurements, previous studies concentrated mainly on measuring the CMCT, and several investigators reported CMCT reductions after IVMP treatment.10,11,12 While these studies generally found an association between overall disease severity and CMCT, a relation between the change in the clinical motor deficit of the investigated limb and of the corresponding CMCT could not be shown in any of these studies. In the present investigation, CMCT did not change significantly in any of the three patient groups, emphasising the lack of sensitivity of this measure and the lack of a relation between CMCT and conduction deficit.9,18,24 We have previously observed that prolongations of CMCT are related to the disease course (relapsing‐remitting v chronic progressive) but not to the motor deficit in a given patient.13
Our results suggest that IVMP has limited efficacy for acute exacerbations in PP‐MS patients. These findings are of particular clinical interest as methylprednisolone may induce neuronal apoptosis in progressive multiple sclerosis.7 In order to carry out a critical re‐evaluation of the role of IVMP in PP‐MS exacerbations, further studies combining clinical and neurophysiological assessment in a larger number of PP‐MS patients would be needed. It also remains to be determined whether the efficacy of IVMP in these patients depends on the degree of the acute deterioration of pyramidal tract function and whether there is a dose dependent effect of IVMP.
Acknowledgements
AMH is supported by a Swiss national grant for a fellowship in clinical neurophysiology (grant 31‐226). The study was also supported by the Swiss National Science Foundation (grants 3100‐053748.98/1 and 3200B0‐100701).
Abbreviations
CMCT - central motor conduction time
EDSS - extended disability scale score
IVMP - intravenous methylprednisolone
MEP - motor evoked potential
PP‐MS - primary progressive multiple sclerosis
RR‐MS - relapsing‐remitting multiple sclerosis
SP‐MS - secondary progressive multiple sclerosis
TMS - transcranial magnetic stimulation
TST - triple stimulation technique
Footnotes
Competing interests: none declared
References
- 1.Filippini G, Brusaferri F, Sibley W A.et al Corticosteroids or ACTH for acute exacerbations in multiple sclerosis. Cochrane Database Syst Rev 20004Cd001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a meta‐analysis of randomized controlled clinical trials. J Neurol 2000247435–442. [DOI] [PubMed] [Google Scholar]
- 3.Milligan N, Newcombe R, Compston D. A double‐blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry 198750511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grauer O, Offenhausser M, Schmidt J.et al [Glucocorticosteroid therapy in optic neuritis and multiple sclerosis. evidence from clinical studies and practical recommendations]. Nervenarzt 200172577–589. [DOI] [PubMed] [Google Scholar]
- 5.Bergamaschi R, Versino M, Raiola E.et al High‐dose methylprednisolone infusions in relapsing and in chronic progressive multiple sclerosis patients. One year follow‐up. Acta Neurol (Napoli) 19931533–43. [PubMed] [Google Scholar]
- 6.Lyons P, Newman P, Saunders M. Methylprednisolone therapy in multiple sclerosis: a profile of adverse effects. J Neurol Neurosurg Psychiatry 198851285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diem R, Hobom M, Maier K.et al Methylprednisolone increases neuronal apoptosis during autoimmune CNS inflammation by inhibition of an endogenous neuroprotective pathway. J Neurosci 2003236993–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker A, Jalinous R, Freeston I. Non‐invasive magnetic stimulation of human motor cortex. Lancet 198511106–1107. [DOI] [PubMed] [Google Scholar]
- 9.Hess C, Mills K, Murray N.et al Magnetic brain stimulation: central motor conduction studies in multiple sclerosis. Ann Neurol 198722744–752. [DOI] [PubMed] [Google Scholar]
- 10.Fierro B, Salemi G, Brighina F.et al A transcranial magnetic stimulation study evaluating methylprednisolone treatment in multiple sclerosis. Acta Neurol Scand 2002105152–157. [DOI] [PubMed] [Google Scholar]
- 11.Kandler R, Jarratt J, Davies‐Jones G.et al The role of magnetic stimulation as a quantifier of motor disability in patients with multiple sclerosis. J Neurol Sci 199110631–34. [DOI] [PubMed] [Google Scholar]
- 12.Salle J, Hugon J, Tabaraud F.et al Improvement in motor evoked potentials and clinical course post‐steroid therapy in multiple sclerosis. J Neurol Sci 1992108184–188. [DOI] [PubMed] [Google Scholar]
- 13.Humm A, Magistris M, Truffert A.et al Central motor conduction differs between acute relapsing‐remitting and chronic progressive multiple sclerosis. Clin Neurophysiol 20031142196–2203. [DOI] [PubMed] [Google Scholar]
- 14.La Mantia L, Riti F, Milanese C.et al Serial evoked potentials in multiple sclerosis bouts. relation to steroid treatment. Ital J Neurol Sci 199415333–340. [DOI] [PubMed] [Google Scholar]
- 15.Smith K, Mcdonald W. The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci 19993541649–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magistris M, Rösler K, Truffert A.et al Transcranial stimulation excites virtually all motor neurons supplying the target muscle. A demonstration and a method improving the study of motor evoked potentials. Brain 1998121437–450. [DOI] [PubMed] [Google Scholar]
- 17.Rösler K, Petrow E, Mathis J.et al Effect of discharge desynchronization on the size of motor evoked potentials: an analysis. Clin Neurophysiol 20021131680–1687. [DOI] [PubMed] [Google Scholar]
- 18.Magistris M, Rosler K, Truffert A.et al A clinical study of motor evoked potentials using a triple stimulation technique. Brain 1999122265–279. [DOI] [PubMed] [Google Scholar]
- 19.Humm A, Beer S, Kool J.et al Quantification of Uhthoff's phenomenon in multiple sclerosis: a magnetic stimulation study. Clin Neurophysiol 20041152493–2501. [DOI] [PubMed] [Google Scholar]
- 20.Mcdonald W, Compston A, Edan G.et al Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 200150121–127. [DOI] [PubMed] [Google Scholar]
- 21.Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell J, Hallett M, Berardelli A.et al Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 19995297–103. [PubMed] [Google Scholar]
- 23.Rossini P, Di Stefano E, Stanzione P. Nerve impulse propagation along central and peripheral fast conducting motor and sensory pathways in man. Electroencephalogr Clin Neurophysiol 198560320–334. [DOI] [PubMed] [Google Scholar]
- 24.Bühler R, Magistris M, Truffert A.et al The triple stimulation technique to study central motor conduction to the lower limbs. Clin Neurophysiol 2001112938–949. [DOI] [PubMed] [Google Scholar]
- 25.Jones S, Brusa A. Neurophysiological markers of relapse, remission and long‐term recovery processes in MS. Electroencephalogr Clin Neurophysiol Suppl 199950584–590. [PubMed] [Google Scholar]
- 26.Smith K J. Conduction properties of central demyelinated and remyelinated axons, and their relation to symptom production in demyelinating disorders. Eye 19948224–237. [DOI] [PubMed] [Google Scholar]
- 27.Andersson P, Goodkin D. Glucocorticosteroid therapy for multiple sclerosis: a critical review. J Neurol Sci 199816016–25. [DOI] [PubMed] [Google Scholar]
- 28.Revesz T, Kidd D, Thompson A.et al A comparison of the pathology of primary and secondary progressive multiple sclerosis. Brain 1994117759–765. [DOI] [PubMed] [Google Scholar]
- 29.Filippi M, Campi A, Martinelli V.et al Comparison Of triple dose versus standard dose gadolinium‐DTPA for detection of MRI enhancing lesions in patients with primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 199559540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson A J, Kermode A G, Macmanus D G.et al Pathogenesis Of progressive multiple sclerosis. Lancet 1989i1322–1323. [DOI] [PubMed]
- 31.Thompson A J, Kermode A G, Wicks D.et al Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol 19912953–62. [DOI] [PubMed] [Google Scholar]
- 32.Kidd D, Barker G J, Tofts P S.et al The transverse magnetisation decay characteristics of longstanding lesions and normal‐appearing white matter in multiple sclerosis. J Neurol 1997244125–130. [DOI] [PubMed] [Google Scholar]