Abstract
Objective
To assess whether different types of malformation of cortical development (MCD) are associated with specific patterns of hippocampal abnormalities.
Methods
A total of 122 consecutive patients with MRI diagnosis of MCD (53 males, age range 1–58 years) were included in the study. Hippocampal measurements were made on 1–3 mm coronal T1‐weighted MRIs and compared with MRIs of normal controls.
Results
A total of 39 patients had focal cortical dysplasia, 5 had hemimegalencephaly, 5 had lissencephaly‐agyria‐pachygyria, 11 had SLH, 11 had PNH, 12 had bilateral contiguous PNH, 5 had schizencephaly, and 34 had polymicrogyria. The frequency of hippocampal abnormalities in these patients with MCD was 29.5%. A small hippocampus was present in all types of MCD. Only patients with lissencephaly and SLH had an enlarged hippocampus. Abnormalities in hippocampal rotation and shape were present in all types of MCD; however, these predominated in PNH. None of the patients with lissencephaly‐agyria‐pachygyria or SLH had hyperintense signal on T2 or FLAIR images or abnormal hippocampal internal architecture.
Conclusion
A small hippocampus was present in all types of MCD; however, the classic MRI characteristics of hippocampal sclerosis were often lacking. Abnormal enlargement of the hippocampus was associated with only diffuse MCD due to abnormal neuronal migration (lissencephaly‐agyria‐pachygyria and SLH).
Keywords: cortical dysgenesis, hippocampus, magnetic resonance imaging, malformations of cortical development, volumetric measurements
Malformations of cortical development (MCD) are congenital anomalies that may develop during different stages of brain development, resulting in various types of cortical abnormality.1 There have been reports of hippocampal abnormalities associated with MCD,2,3,4,5,6,7,8,9,10 however, no particular pattern of hippocampal abnormality has been found to be associated with a specific type of cortical lesion. Volumetric measurements enable the identification of subtle hippocampal abnormalities that are usually missed by routine visual inspection.11,12,13,14,15
This descriptive study aimed to assess the frequency and characteristics of hippocampal abnormalities associated with MCD, and whether a particular type of hippocampal abnormality is associated with the different forms of MCD. In addition, we investigated the association between MCD and hippocampal abnormalities with frequency of seizures.
Patients and methods
Patients
We evaluated 122 consecutive patients with the diagnosis of MCD defined by high resolution magnetic resonance imaging (MRI). The indications for MRI were diverse and included motor or language developmental delay, mental retardation, seizures, or focal neurological deficits. We used the criteria proposed by Barkovich et al1 for diagnosis and classification of MCD. The diagnosis of MCD was made in a prospective manner and included all types of MCD in these consecutive patients, although we analysed the data retrospectively. A total of 90 patients were from the University of Campinas (UNICAMP) and 32 were from the Montreal Neurological Institute (MNI) (53 males, 69 females; mean age 21.1 years, range 1–58). All patients gave informed consent according to the declaration of Helsinki, and the protocol was approved by the ethical committee of both institutions.
We analysed the data according to the type of lesion: focal cortical dysplasia, isolated single or multiple periventricular heterotopia (PNH), bilateral contiguous PNH, lissencephaly‐agyria‐pachygyria, subcortical laminar heterotopia (SLH), polymicrogyria, schizencephaly, and hemimegalencephaly.
MRI
For MRI we used a 2.0 T (Elscint Prestige, Haifa, Israel) or 1.5 T ACS III Gyroscan (Philips Medical Systems, Best, the Netherlands) as follows:
Sagittal T1 spin‐echo for optimal orientation of the subsequent images
3 mm thick coronal T1 inversion recovery (T1‐IR) and T2‐weighted images
Axial T1‐weighted and fluid attenuated inversion recovery (FLAIR) images
Volumetric (three dimensional (3D)) T1 gradient echo (T1‐GRE), 1–1.5 mm thick, acquired in the sagittal plane for multiplanar reconstruction
Volumetry
Measurements of hippocampal formation were made according to a standardised protocol.12,13,14 We used the 3 mm T1‐IR images or 1–1.5 mm 3D T1‐GRE images in the coronal plane for manual contouring following anatomical guidelines. To determine the presence of unilateral or bilateral symmetrical or asymmetrical hippocampal abnormalities, the final hippocampal volume was corrected either by the variation in the total brain volume12 or by measuring hippocampi in normalised scans as has been previously described.14
We compared the hippocampal volumes of the 92 patients older than 13 years with those of a control group of young healthy volunteers (n = 30) from each institution (UNICAMP: mean age 32 years, range 8–52; MNI: mean age 29.5 years, range 20–56). Hippocampal volumes of the 30 patients younger than 13 years were compared with those of a control group of 19 children (mean age 6.7 years, range 1–13). These 19 children had undergone MRI for investigation of headache, possible developmental delay, or learning difficulties. All the MRI had normal findings and neurological examination was also normal. These children had been followed up for a minimum of 2 years after the MRI. Therefore, they were considered normal controls “a posteriori”. Values lesser than or greater than two standard deviations from the mean of the respective control group were considered abnormal. To allow comparisons of volume abnormalities from the two different protocols, we transformed all data into z scores (standardised scores which express the number of standard deviations away from the mean of the appropriate control group).
Hippocampal rotation, shape, internal architecture, and signal abnormalities
In addition to volumetric studies, we performed careful systematic visual analysis of all MRIs, including the T1‐weighted and T2‐weighted images. Visual assessment of hippocampal integrity took into account hippocampal signal, internal structure, and shape of the hippocampi and other mesial temporal structures.4 We analysed the images on a Silicon Graphics workstation (Moutain View, CA) with imaging post‐processing software (Omnipro2, Picker International Inc.; or Display program, www.bic.mni.mcgill.ca), which allows changing windowing (contrast/brightness) and realignment of images as well as multiplanar reconstruction. We paid particular attention to the form of the hippocampi along their entire axis as well as to the morphology of adjacent mesial temporal structures. This analysis was performed separately from the volumetry and was crosschecked by at least two investigators experienced in evaluating images of patients with cortical dysgenesis.
We defined abnormal shape and rotation as well as loss of internal structure based on visual analysis, comparing with the normal appearance of hippocampi from our MRI normal control database consisting of 50 healthy volunteers (normal controls “a priori”; age range 17–58 years) from each institution (n = 100), and the group of 19 children described above (normal controls “a posteriori”). The following features, as proposed by Baulac et al,2 were considered as signs of abnormal shape or rotation of hippocampi:
a medial position of the hippocampus close to the midline
hippocampus that was abnormally round or pyramidal in shape and vertically oriented instead of being ovoid and transversely oriented.
Results
A total of 39 patients had focal cortical dysplasia, 5 had hemimegalencephaly, 5 had lissencephaly‐agyria‐pachygyria, 11 had SLH, 11 had PNH, 12 had bilateral contiguous PNH, 5 had schizencephaly, and 34 had polymicrogyria. The frequency of hippocampal abnormalities in these patients with MCD was 29.5% (36/122; tables 1 and 2).
Table 1 Summary of MRI findings in patients with hippocampal abnormalities.
| ID | Age/sex | Group | Lesion side | Volume | Shape | Internal architecture | Rotation | ↑ Signal on T2/FLAIR |
|---|---|---|---|---|---|---|---|---|
| 1 | 19/F | FCD | R | ↓ R | Normal | Abnormal R | Normal | ↑ R |
| 2 | 29/F | FCD | R | ↓ R | Round R | Abnormal R | Normal | ↑ R |
| 3 | 16/M | FCD | L | ↓ L | Normal | Abnormal L | Abnormal L | ↑ L |
| 4 | 18/M | FCD | R | ↓ R | Normal | Normal | Normal | ↑ R |
| 5 | 18/M | FCD | L | ↓ L | Round L | Abnormal L | Normal | Normal |
| 6 | 37/M | HME | R | ↑ R | Normal | Abnormal R | Normal | ↑ R |
| 7 | 10/F | HME | R | ↓ Bilateral | Normal | Normal | Normal | Normal |
| 8 | 1/F | HME | R | ↑ R | Normal | Normal | Abnormal R | Normal |
| 9 | 1/F | HME | R | ↓ R | Round bilateral | Normal | Abnormal bilateral | Normal |
| 10 | 1/2 | HME | R | ↓ Bilateral | Normal | Normal | Normal | Normal |
| 11 | 18/F | SLH | Bilateral | ↑ Bilateral | Normal | Normal | Normal | Normal |
| 12 | 29/M | SLH | Bilateral | ↓ L | Round bilateral | NA | Abnormal L | NA |
| 13 | 32/M | SLH | Bilateral | ↓ Bilateral | Round bilateral | NA | Abnormal bilateral | NA |
| 14 | 19/F | SLH | Bilateral | ↓ R | Normal | NA | Normal | NA |
| 15 | 36/M | Ag‐P | Bilateral | ↑ Bilateral | Round bilateral* | Normal | Normal | Normal |
| 16 | 4/F | Ag‐P | Bilateral | ↑ Bilateral | Normal | Normal | Normal | Normal |
| 17 | 50/M | PNH | L | ↓ Bilateral (L>R) | Abnormal L, round R | Abnormal L | Abnormal bilateral* | Normal |
| 18 | 19/F | PNH | R | ↓ Bilateral (R>L) | Round bilateral* | Normal | Abnormal bilateral | Normal |
| 19 | 38/M | PNH | R | ↓ L | Normal | Abnormal L | Abnormal L | NA |
| 20 | 40/M | PNH | Bilateral | ↓ R | Round R | Abnormal bilateral | Abnormal bilateral | ↑ Bilateral |
| 21 | 8/F | PNH | R | ↓ R | Round R | Abnormal R | Abnormal R | ↑ R |
| 22 | 16/M | PNH | Bilateral | ↓ L | Round bilateral | Abnormal bilateral | Abnormal bilateral | NA |
| 23 | 31/F | BPNH | Bilateral | ↓ R | Round bilateral | Abnormal bilateral | Abnormal bilateral | ↑ Bilateral |
| 24 | 16/F | BPNH | Bilateral | ↓ R | Round L | Abnormal R | Abnormal bilateral | ↑ Bilateral |
| 25 | 35/F | BPNH | Bilateral | ↓ R | Round bilateral | Abnormal bilateral | Abnormal bilateral | ↑ Bilateral |
| 26 | 28/M | BPNH | Bilateral | ↓ L | Round L | Abnormal L | Abnormal bilateral | ↑ bilateral |
| 27 | 17/M | SCZ | R | ↓ Bilateral (R>L) | Round bilateral | Abnormal bilateral | Abnormal bilateral | Normal |
| 28 | 8/F | SCZ | R* | ↓ Bilateral (R>L) | Round R*, flat L | Abnormal R | Normal | ↑ L |
| 29 | 37/M | SCZ | R | ↓ R | Round R | Abnormal R | Normal | Normal |
| 30 | 20/M | PMG | R | ↓ Bilateral | Normal | Normal | Normal | Normal |
| 31 | 6/M | PMG | Bilateral | ↓ Bilateral | Round bilateral | Normal | Normal | Normal |
| 32 | 4/M | PMG | Bilateral | ↓ Bilateral | Normal | Normal | Normal | Normal |
| 33 | 5/F | PMG | Bilateral | ↓ L | Round bilateral | Normal | Abnormal bilateral | Normal |
| 34 | 8/M | PMG | Bilateral | ↓ L | Normal | Normal | Normal | Normal |
| 35 | 30/F | PMG | Bilateral | ↓ R | Normal | Normal | Normal | NA |
| 36 | 41/M | PMG | Bilateral | ↓ L | Normal | Normal | Normal | Normal |
Ag‐P, agyria‐pachygyria/lissencephaly; BPNH, bilateral periventricular nodular heterotopia; F, female; FCD, focal cortical dysplasia; HME, hemimegalencephaly; L, left; M, male; PNH, isolated single or multiple unilateral periventricular heterotopia; PMG, polymicrogyria; R, right; SLH, subcortical laminar heterotopia; SCZ, schizencephaly.
Table 2 Grouped data summarising the frequency of hippocampal abnormalities in each type of malformation of cortical development (MCD).
| Type of MCD | Hippocampal atrophy (n (%)) | Enlarged hippocampus (n (%)) | Abnormal rotation (n (%)) | Abnormal shape (n (%)) | Abnormal internal architecture (n (%)) | Abnormal T2/FLAIR signal (n (%)) |
|---|---|---|---|---|---|---|
| FCD (n = 39) | 5 (13) | 0 | 1 (2.5) | 2 (5) | 4 (10) | 4 (10) |
| HME (n = 5) | 3 (60) | 2 (40) | 2 (40) | 1 (20) | 1 (20) | 1 (20) |
| Ag‐P (n = 5) | 0 | 2 (40) | 0 | 1 (20) | 0 | 0 |
| SLH (n = 11) | 3 (27) | 1 (9) | 2 (18) | 2 (18) | 0 | 0 |
| PNH (n = 11) | 6 (54.5) | 0 | 6 (54.5) | 5 (45.5) | 5 (45.5) | 2 (18) |
| BPNH (n = 12) | 4 (33) | 0 | 4 (33) | 4 (33) | 4 (33) | 4 (33) |
| SCZ (n = 5) | 3 (60) | 0 | 1 (20) | 3 (60) | 3 (60) | 1 (20) |
| PMG (n = 34) | 7 (20.5) | 0 | 1 (3) | 2 (6) | 0 | 0 |
| Total (n = 122) | 31 (25.5) | 5 (4) | 17 (14) | 20 (16.5) | 17 (14) | 12 (10) |
Two patients with HME with enlarged hippocampus also had pachygyria in the temporal lobe.
For abbreviations see table 1 footnote.
Small hippocampus
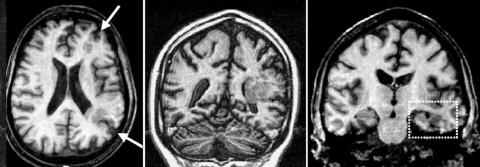
The number of patients with a small hippocampus (fig 1) in each MCD group is given in table 2. In all but one patient the hippocampal abnormality was ipsilateral to the cortical lesion.
Figure 1 Middle panel: Coronal T1‐weighted image showing unilateral periventricular heterotopia. Note that the cortex above the lesion is abnormal, and probably contains areas of polymicrogyria. Left panel: Axial T1‐weighted image showing that the lesion involves mostly watershed areas at the frontal and occipital lobes (arrows). Right panel: Coronal T1‐weighted image showing atrophy of the left hippocampal formation (box).
Enlarged hippocampus
Two patients with lissencephaly‐agyria‐pachygyria, one patient with SLH (table 2), and two patients with hemimegalencephaly had enlarged hippocampi. However, both the patients with hemimegalencephaly also had pachygyria in the temporal lobe (fig 2).
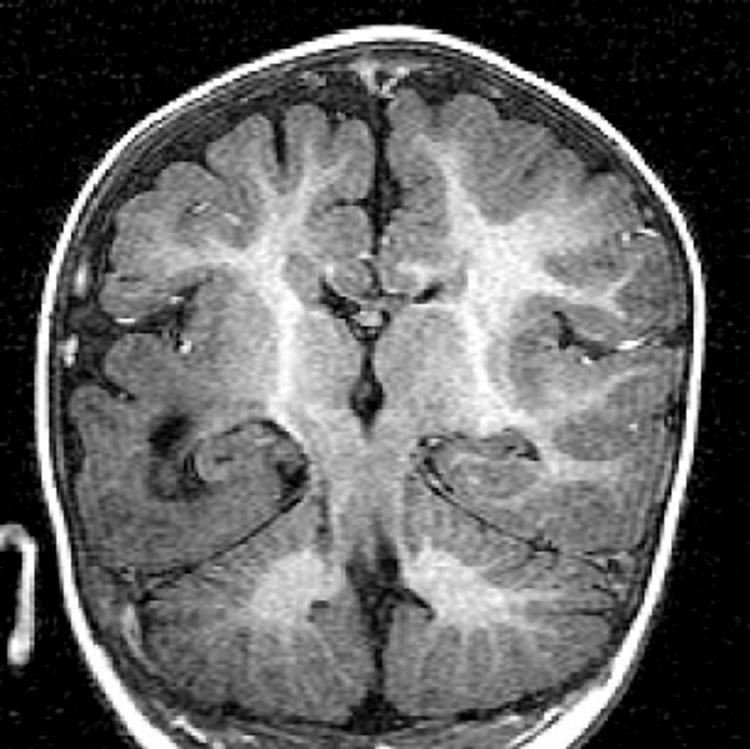
Figure 2 Coronal T1 image showing an enlarged right hippocampus. Note that the temporal lobe has thickened cortex and abnormal gyration compatible with lissencephaly/agyria‐pachygyria.
Hippocampal rotation, shape, internal architecture, and T2‐FLAIR signal
Abnormalities in hippocampal rotation and shape were present in all types of MCD; however, they predominated in patients with PNH (see table 2). None of the patients with lissencephaly‐agyria‐pachygyria or SLH presented a hyperintense signal on T2 or FLAIR images or abnormalities of the internal architecture of the hippocampus (tables 1 and 2).
Epilepsy
A total of 102 (84%) patients had epilepsy. Three patients with small hippocampi had never had seizures. All three had perisylvian polymicrogyria (patients 31, 32, and 33 in table 1). Only four patients had had febrile seizures during childhood: three with focal cortical dysplasia and one with periventricular heterotopia. Only one of these four patients had hippocampal atrophy.
Discussion
We found that small hippocampi were present in all types of MCD. Although the cause of hippocampal atrophy has not been established, complex febrile seizures, status epilepticus, and genetic predisposition are the most likely aetiological factors.16,17,18,19,20,21 The commonest type of extrahippocampal lesion found in dual pathology has been MCD.5,6,9,15
The issue of prolonged febrile seizures occurring early in life, leading to hippocampal atrophy and temporal lobe epilepsy, still remains controversial. A subtle pre‐existing hippocampal malformation may facilitate febrile seizures and contribute to the development of subsequent hippocampal atrophy.22 However, only four of our patients had febrile seizures during childhood, and three of these four had normal hippocampal volumes. These observations support the notion that the relation between febrile seizures and hippocampal atrophy is more complex than previously thought.19,21,22,23 Status epilepticus is a well documented cause of hippocampal atrophy; however, it is still unclear if habitual brief partial seizures can cause progressive hippocampal atrophy in patients with intractable temporal lobe epilepsy, or if they play a role in the progression of previously existing hippocampal pathology.16,17,18,23,24,25 Several lines of evidence indicate that the interaction of multiple factors, rather than only the occurrence of partial seizures, may be necessary for development of full‐blown hippocampal atrophy and other signs of hippocampal sclerosis.2,9,10,15,16,17,18,19,20,21,22,23,24,25,26,27 In addition, hippocampal atrophy may be present in individuals who have never had seizures.16 In fact, three of our patients with MCD and hippocampal atrophy had never had seizures. A vascular injury is likely to be involved in the pathogenesis of some forms of MCD.28,29 Unilateral PNH usually involves watershed areas in the frontal and occipital–temporal regions, and sometimes these lesions are associated with ipsilateral hippocampal atrophy (see fig 1). This raises the possibility that, in such patients, both lesions are caused by vascular injury during early development.9
Proportionally enlarged hippocampi were present only in patients with lissencephaly‐agyria‐pachygyria and SLH. The hippocampal enlargement was always bilateral and symmetrical. This feature may be missed during visual inspection because the temporal horns of the lateral ventricles are also usually enlarged, giving the appearance of a small hippocampus.30 Modified cell death has been proposed as having a role in the genesis of MCD due to abnormal cellular proliferation/apoptosis and neuronal migration.1,31 Although the pathogenesis of the abnormally enlarged hippocampi remains unclear, perhaps abnormal apoptosis could result in an excess of neurones, and consequently in an enlarged hippocampus. However, it is difficult to evaluate the histopathological spectrum of abnormally enlarged hippocampi, because focal surgical resection is rarely indicated in these patients.
In the present series, hemimegalencephaly was associated with either a small or an enlarged hippocampus. This may be explained by the fact that this malformation results from abnormalities during all three fundamental stages of cortical development: cellular proliferation/apoptosis, neuronal migration, and cortical organisation.1 The enlarged hippocampi were observed when abnormalities of proliferation/apoptosis or migration predominated in the temporal lobe, as illustrated in fig 2. Conversely, when abnormalities of cortical organisation predominated, we found a small hippocampus.
Detailed visual analysis of the MRI characteristics of hippocampal abnormalities in our patients revealed that the atrophy present in these patients was not always associated with hyperintense signal or with an abnormal internal structure of the hippocampus (table 1). This is in keeping with the concept of a spectrum of hippocampal sclerosis in these patients.32 Hippocampal rotation and shape has not previously been systematically evaluated in a large series of patients with MCD. We found that abnormalities of hippocampal rotation and shape were present in all types of MCD; however, they predominated in patients with PNH. None of the patients with lissencephaly‐agyria‐pachygyria and SLH had a hyperintense signal on T2 or FLAIR images or abnormalities in the internal architecture of the hippocampus. The clinical significance of abnormalities of rotation and shape of the hippocampus is not always clear since this type of change is sometimes seen in otherwise normal individuals.16
We conclude that developmental abnormalities of the hippocampus are frequently present in patients with MCD. Our study not only demonstrated atrophy but also in some instances revealed enlargement of the hippocampus, which has not been previously described. This raises the question of the significance of abnormal rotation and shape of the hippocampus. A small hippocampus was encountered in patients with all types of MCD; however, they often lacked other classic MRI signs of hippocampal sclerosis. Abnormally enlarged hippocampi were found in diffuse MCD due to abnormal neuronal migration (lissencephaly‐agyria‐pachygyria and SLH) and were not associated with hyperintense T2 signal or loss of internal hippocampal architecture.
Abbreviations
FLAIR - fluid attenuated inversion recovery
MCD - malformation of cortical development
MRI - magnetic resonance imaging
PNH - periventricular nodular heterotopia
SLH - subcortical laminar heterotopia
Footnotes
Supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil (grant no. 97/07584‐3 to Dr Cendes, and 00/04710‐2 to Dr Li). Dr Montenegro received a scholarship from FAPESP (grant no. 00/03502‐7). Dr Kinay received a scholarship from the Savoy Foundation for Epilepsy Research, Montreal, Quebec, Canada.
Competing interests: none declared
References
- 1.Barkovich A J, Kuzniecky R I, Jackson G D.et al Classification system for malformations of cortical development—update 2001. Neurology 2001572168–2178. [DOI] [PubMed] [Google Scholar]
- 2.Baulac M, De Grissac N, Hasboun D.et al Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 199844223–233. [DOI] [PubMed] [Google Scholar]
- 3.Ho S S, Kuzniecky R I, Gilliam F.et al Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology 199850748–754. [DOI] [PubMed] [Google Scholar]
- 4.Kuzniecky R I, Jackson G D.Magnetic resonance in epilepsy. New York: Raven Press, 1995
- 5.Lévesque M F, Nakasato N, Vinters H V.et al Surgical treatment of limbic epilepsy associated with extrahippocampal lesions: the problem of dual pathology. J Neurosurgery 199175364–370. [DOI] [PubMed] [Google Scholar]
- 6.Li L M, Cendes F, Andermann F.et al Surgical outcome in patients with epilepsy and dual pathology. Brain 1999122799–805. [DOI] [PubMed] [Google Scholar]
- 7.Prayson R A, Reith J D, Najm I M. Mesial temporal sclerosis. A clinicopathologic study of 27 patients, including 5 with coexistent cortical dysplasia. Arch Pathol Lab Med 1996120532–536. [PubMed] [Google Scholar]
- 8.Prayson R A, Bingaman W, Frater J L.et al Histopathologic findings in 37 cases of functional hemispherectomy. Ann Diagn Pathol 19993205–212. [DOI] [PubMed] [Google Scholar]
- 9.Raymond A A, Fish D R, Stevens J M.et al Association of hippocampal sclerosis with cortical dysgenesis in patients with epilepsy. Neurology 1994441841–1845. [DOI] [PubMed] [Google Scholar]
- 10.Vernet O, Farmer J P, Montes J L.et al Dysgenetic mesial temporal sclerosis: an unrecognized entity. Childs Nerv Syst 200016719–723. [DOI] [PubMed] [Google Scholar]
- 11.Cascino G D, Jack C R, Jr, Parisi J E.et al Magnetic resonance imaging‐based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol 19913031–36. [DOI] [PubMed] [Google Scholar]
- 12.Cendes F, Andermann F, Gloor P.et al MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 199343719–725. [DOI] [PubMed] [Google Scholar]
- 13.Watson C, Jack C R, Cendes F. Volumetric MRI: clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol 1997541521–1531. [DOI] [PubMed] [Google Scholar]
- 14.Bernasconi N, Bernasconi A, Caramanos Z.et al Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 2003126462–469. [DOI] [PubMed] [Google Scholar]
- 15.Cendes F, Cook M J, Watson C.et al Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology 1995452058–2064. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi E, Li L M, Lopes‐Cendes I.et al Magnetic resonance imaging evidence of hippocampal sclerosis in asymptomatic, first‐degree relatives of patients with familial mesial temporal lobe epilepsy. Arch Neurol . 2002;591891–1894. [DOI] [PubMed]
- 17.Kälviäinen R, Salmenperä T, Partanen K.et al Recurrent seizures in temporal lobe epilepsy. Neurology . 1998;501377–1382. [DOI] [PubMed]
- 18.Kobayashi E, Lopes‐Cendes I, Guerreiro C A M.et al Seizure outcome and hippocampal atrophy in familial mesial temporal lobe epilepsy. Neurology 200156166–172. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Ebner A. Prolonged febrile convulsions and mesial temporal lobe epilepsy in an identical twin. Neurology 200157318–320. [DOI] [PubMed] [Google Scholar]
- 20.Sutula T P, Pitkänen A. More evidence for seizure‐induced neuron loss: Is hippocampal sclerosis both cause and effect of epilepsy? Neurology 200157169–170. [DOI] [PubMed] [Google Scholar]
- 21.Van Landigham K E, Heinz E R, Cavazos J E.et al Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol 199843413–426. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez G, Effenberger O, Vinz B.et al Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis. Neurology 199850909–917. [DOI] [PubMed] [Google Scholar]
- 23.Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol 200417161–164. [DOI] [PubMed] [Google Scholar]
- 24.Gorter J A, Gonçalves‐Pereira P M, van Vliet E A.et al Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia 200344647–658. [DOI] [PubMed] [Google Scholar]
- 25.Germano I M, Sperber E F, Ahuja S.et al Evidence of enhanced kindling and hippocampal neuronal injury in immature rats with neuronal migration disorders. Epilepsia 1998121253–1260. [DOI] [PubMed] [Google Scholar]
- 26.Franceschi M, Triulzi F, Ferini‐Strambi L.et al Focal cerebral lesions found by magnetic resonance imaging in cryptogenic nonrefractory temporal lobe epilepsy patients. Epilepsia 198930540–546. [DOI] [PubMed] [Google Scholar]
- 27.Tasch E, Cendes F, Li L M.et al Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol 199945568–576. [DOI] [PubMed] [Google Scholar]
- 28.Barkovich A J, Kjos B O. Schizencephaly: correlation of clinical findings with MRI characteristics. AJNR Am J Neuroradiol 19921385–94. [PMC free article] [PubMed] [Google Scholar]
- 29.Toti P, De Felice C, Palmeri M L D.et al Inflammatory pathogenesis of cortical polymicrogyria: an autopsy study. Pediatr Res 199844291–296. [DOI] [PubMed] [Google Scholar]
- 30.Baker L L, Barkovich A J. The large temporal horn: MR analysis in developmental brain anomalies versus hydrocephalus. AJNR Am J Neuroradiol 199213115–122. [PMC free article] [PubMed] [Google Scholar]
- 31.Mischel P S, Nguyen L P, Vinters H V. Cerebral cortical dysplasia associated with paediatric epilepsy. Review of neuropathological features and proposal for a grading system. J Neuropathol Exp Neurol 199554137–153. [DOI] [PubMed] [Google Scholar]
- 32.Van Paesschen W, Connelly A, King M D.et al The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol 19974141–51. [DOI] [PubMed] [Google Scholar]