Abstract
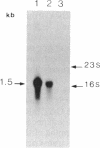
Spiroplasmas are wall-less procaryotes in which the UGA codon serves not as a stop signal but as a code for the amino acid tryptophan. Spiroplasma genes that contain UGA codons thus cannot be studied in the usual Escherichia coli cloning and expression systems. Although this problem can be circumvented by using UGA-suppressor strains of E. coli, spiroplasmas themselves would provide a more efficient cloning and expression host. We have now successfully employed the replicative form (RF) of a filamentous spiroplasma virus (SpV1) to clone and express the E. coli-derived chloramphenicol acetyltransferase (CAT) gene in Spiroplasma citri. The CAT gene was inserted in one of the four intergenic regions of the SpV1 RF and introduced into cells by electroporation. Both the RF and the virion DNA produced by the transfected cells contained the CAT gene sequences. Northern blot analysis, primer extension, and S1 mapping showed that transcription of the CAT gene started from a promoter located on the SpV1 RF and was terminated downstream of the CAT gene, still within the viral RF. Expression of the CAT gene was demonstrated by acetylation of chloramphenicol by cell-free extracts from the transfected spiroplasmas.
Full text
PDF

Images in this article
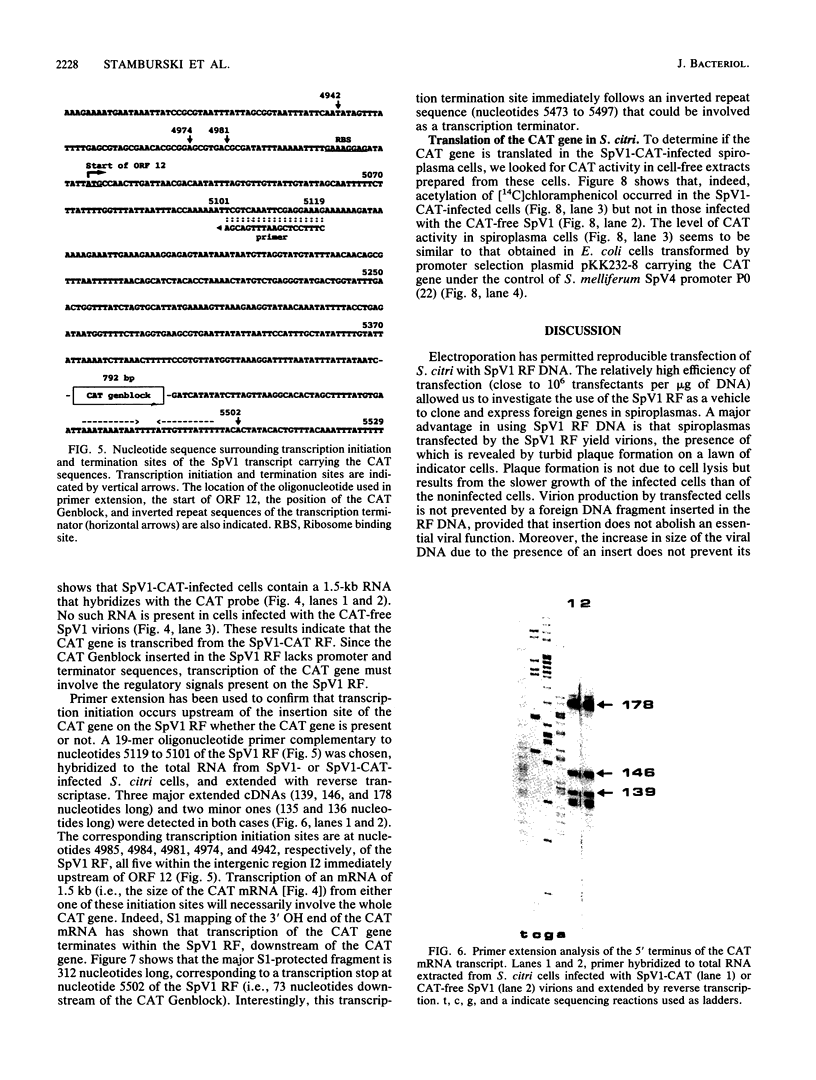
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C., Saillard C., Bové J. M. Organization and nucleotide sequences of the Spiroplasma citri genes for ribosomal protein S2, elongation factor Ts, spiralin, phosphofructokinase, pyruvate kinase, and an unidentified protein. J Bacteriol. 1990 May;172(5):2693–2703. doi: 10.1128/jb.172.5.2693-2703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dybvig K., Cassell G. H. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science. 1987 Mar 13;235(4794):1392–1394. doi: 10.1126/science.3029869. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascarel-Devilder M. C., Renaudin J., Bove J. M. The spiroplasma virus 4 replicative form cloned in Escherichia coli transfects spiroplasmas. Virology. 1986 Jun;151(2):390–393. doi: 10.1016/0042-6822(86)90060-7. [DOI] [PubMed] [Google Scholar]
- Renaudin J., Aullo P., Vignault J. C., Bové J. M. Complete nucleotide sequence of the genome of Spiroplasma citri virus SpV1-R8A2 B. Nucleic Acids Res. 1990 Mar 11;18(5):1293–1293. doi: 10.1093/nar/18.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin J., Pascarel M. C., Bove J. M. Cloning and sequencing of the genome of spiroplasma virus 4. Isr J Med Sci. 1987 May;23(5):427–428. [PubMed] [Google Scholar]
- Renaudin J., Pascarel M. C., Bové J. M. Spiroplasma virus 4: nucleotide sequence of the viral DNA, regulatory signals, and proposed genome organization. J Bacteriol. 1987 Nov;169(11):4950–4961. doi: 10.1128/jb.169.11.4950-4961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renbaum P., Abrahamove D., Fainsod A., Wilson G. G., Rottem S., Razin A. Cloning, characterization, and expression in Escherichia coli of the gene coding for the CpG DNA methylase from Spiroplasma sp. strain MQ1(M.SssI). Nucleic Acids Res. 1990 Mar 11;18(5):1145–1152. doi: 10.1093/nar/18.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Maniloff J. Polyethylene glycol-dependent transfection of Acholeplasma laidlawii with mycoplasma virus L2 DNA. J Bacteriol. 1983 Aug;155(2):734–741. doi: 10.1128/jb.155.2.734-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamburski C., Renaudin J., Bove J. M. Characterization of a promoter and a transcription terminator of Spiroplasma melliferum virus SpV4. J Bacteriol. 1990 Oct;172(10):5586–5592. doi: 10.1128/jb.172.10.5586-5592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]