Cholesterol 24S‐hydroxylase (CYP46; Gene ID: 10858) in chromosome 14q32.1 plays a key role in the hydroxylation of brain cholesterol to 24‐hydroxycholesterol.1 This primary cholesterol elimination product can be transported through the blood‐brain barrier. 24S‐hydroxycholesterol is in in vitro conditions neurotoxic and may contribute to neurodegeneration.2
The gene for apolipoprotein E (APOE), a major cholesterol‐transporting plasma protein, is expressed as three different polymorphic allelic forms (APOE ε2, ε3, and ε4) of which APOE ε4 is associated with an increased risk of Alzheimer's disease (AD). A small amount of cholesterol exits via an APOE mediated pathway through the cerebrospinal fluid.1 Because depletion of brain cholesterol levels reduces the generation of β amyloid protein in the brain and cholesterol lowering drugs may reduce the risk of dementia,1 we tested the hypothesis that the previously examined single nucleotide polymorphism dbSNP:754203 in the CYP46 gene3 with or without the APOE ε4 allele was associated with AD in a Finnish population. Further, we performed a meta‐analysis of the CYP46 dbSNP:754203 polymorphism using all reported findings of similar association studies on Medline (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi) up to April 2005 to evaluate the true global effect of the polymorphism as a susceptibility factor for AD.
The study population was examined in the Department of Neurology, Kuopio University Hospital.4 The ethical committee of University Hospital approved the study. The subjects consisted of 422 AD patients (mean age of onset 72±7 years; 293 (69%) women) and 469 controls (mean age at examination or death 70±5 years; 284 (61%) women), who had no signs of cognitive decline on interview or neuropsychological testing. There was a positive family history of AD in 36% of AD patients, but inconclusive evidence of autosomal dominant transmission. If a reliable family history was not available, the disease was termed sporadic. All AD subjects underwent a comprehensive clinical evaluation during which a clinical diagnosis of probable AD was made according to the NINCDS‐ADRDA criteria. There were 68 AD patients (16% of all AD cases) with onset at 65 years of age or below, 63 of whom were screened for known AD mutations in APP, PSEN‐1, or PSEN‐2 genes. Although no such mutations were found, it is still possible that some of these patients may carry rare variants of these genes.
We genotyped dbSNP:754203 T to C substitution in intron 2 by using the ABI PRISM SNaPshot Multiplex assay and ABI 3100 Genetic Analyzer (http://appliedbiosystems.com). Amplification primers for dbSNP:754203 have been described previously3; the following specific primer was used in a SNaPshot reaction with the length of the tail indicated by the subscript: 5′‐T31CAACAGGGCAGAGCCTTGCCCCC‐3′. Genotype frequencies were in Hardy‐Weinberg equilibrium in cases and controls. APOE genotyping was determined by a standard method.4 Meta‐analysis was carried out with RevMan 4.2 software (http://www.cc‐ims.net/RevMan/). Fixed and random effects meta‐analyses were performed depending on the heterogeneity of the effects. Pooled and individual study effects were estimated by applying a maximum likelihood estimation technique. Binary logistic regression analyses were carried out with SPSS version 11.5 and the level of statistical significance was set at p⩽0.05.
Results
The distribution of APOE ε2, ε3, and ε4 alleles in the AD patients and controls was 0.02/0.51/0.47 and 0.04/0.80/0.16, respectively; this result was consistent with that previously found in the same population.4 The APOE ε4 allele was significantly associated with AD (OR 4.8, 95% CI 3.8 to 6.0; p<0.001) and age at onset was 3 years earlier for ε4+ versus ε4− patients (71 v 74 years of age, p<0.001). In logistic regression tests, the subjects were also divided on the basis of APOE genotype into APOE4 carriers and non‐carriers.
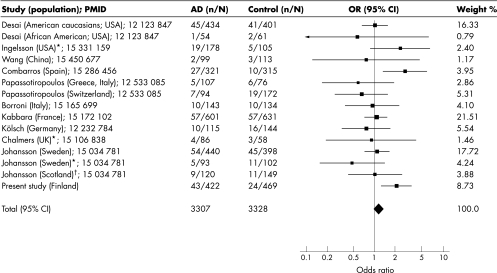
We found a significant difference (p = 0.015) in the distribution of dbSNP:754203 CC, CT, and TT genotypes between all AD subjects and controls. We also observed a significant association between the dbSNP:754203 CC genotype and AD in all and APOE4+ subjects (311 AD patients and 129 controls). The age and sex adjusted odds ratio for the risk of AD in the carriers of the dbSNP:754203 CC genotype was 2.13 (95% CI 1.25 to 3.62; p = 0.005) and 3.58 (95% CI 1.21 to 10.56; p = 0.021) compared with the CT/TT genotypes for all and APOE4+ subjects, respectively. In the subgroup of patients with onset at 65 years of age or below, the distribution of dbSNP:754203 genotypes did not differ significantly from controls, but it did differ in the subgroup with onset at 65 years of age or above. However, the highest significance was achieved when all study material was considered together. For meta‐analysis of dbSNP:754203, 15 case‐control studies including this one were pooled, resulting in 3307 cases and 3328 controls (fig 1). A fixed effects meta‐analysis was performed since the likelihood ratio test did not provide significant evidence of between study heterogeneity (χ2 = 20.49, df 14; p = 0.12). In the pooled analysis of different ethnic populations, a pooled effect of CC genotype frequencies in AD cases was very similar to those found in controls (OR 1.16; 95% CI 0.97 to 1.38).
Figure 1 Meta‐analysis of the dbSNP:754203 CC genotype carriers. OR and 95% CI of the genotype frequencies are shown. *Autopsy confirmed sample; 54 AD cases confirmed (Ingelsson). †Early onset AD sample. n/N, number of CC genotype carriers/number of subjects. Weight %, weighting proportion in an individual study (used when combining ORs). In the separate pooled analysis of USA and European populations, a pooled effect of CC genotype frequencies was similar to those found in controls (OR 1.17; 95% CI 0.78 to 1.73 and OR 1.16; 95% CI 0.95 to 1.41, for USA and European subjects, respectively). Hardy‐Weinberg equilibrium was not re‐examined in studies for meta‐analysis. PMID, PubMed ‐ indexed for Medline (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi). We did not show allele frequencies of this SNP because the Alzheimer Research Forum (http://www.alzforum.org/home.asp) has previously published OR and 95% CI of allele frequencies of dbSNP:754203 in their meta‐analysis.
Comment
This candidate gene based association study of the CYP46 gene in a large series of AD patients and controls implicated the CYP46 intron 2 CC genotype as a possible risk factor for Finnish AD patients. For maximum power in statistical tests, we primarily used all AD and control material together, but since the APOE polymorphism is an established risk factor for AD we also determined the effect of APOE genotypes on possible CYP46 association with AD.
There are several contradictory studies describing either risk, no association, or even a beneficial effect for dbSNP:754203 and AD (fig 1). Although allele/genotype frequencies of dbSNP:754203 and AD may be population or geographic specific, the meta‐analysis argues against the hypothesis that dbSNP:754203 plays a crucial role in the interaction between the CYP46 gene and AD (fig 1). Since the relevant variation dbSNP:754203 is intronic, it is not clear how it influences the function of the respective enzyme. It may be in linkage disequilibrium with an as yet unidentified functional variant on CYP46 coding exons or promoter region. Interestingly, dbSNP:754203 was not associated with 24S‐hydroxycholesterol/cholesterol ratios in CSF of AD patients.5 However, the dbSNP:754203 polymorphism correlated with increased β amyloid load in brain tissues as well as with increased CSF levels of β amyloid and phosphorylated tau proteins.5 We conclude that the CYP46 intron 2 polymorphism may influence the risk of developing AD in the Finnish population, although meta‐analysis does not support this role on a global scale.
Acknowledgements
The authors are grateful to Ms Petra Mäkinen and Ms Marjo Laitinen for their skilful technical help with the SNP screening and genotyping.
Electronic‐database information
The following URLs have been mentioned in this article: PubMed: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi; Applied Biosystems: http://appliedbiosystems.com; RevMan: http://www.cc‐ims.net/RevMan/; and the Alzheimer Research Forum: http://www.alzforum.org/home.asp.
Copyright © 2006 BMJ Publishing Group
Footnotes
The study was supported by the Health Research Council of the Academy of Finland, an EVO grant (no 5772708) from Kuopio University Hospital, and the Nordic Center of Excellence in Neurodegeneration
Competing interests: none declared
The following URLs have been mentioned in this article: PubMed: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi; Applied Biosystems: http://appliedbiosystems.com; RevMan: http://www.cc‐ims.net/RevMan/; and the Alzheimer Research Forum: http://www.alzforum.org/home.asp.
References
- 1.Raffaï R L, Weisgraber K H. Cholesterol: from heart attacks to Alzheimer's disease. J Lipid Res 2003441423–1430. [DOI] [PubMed] [Google Scholar]
- 2.Kölsch H, Ludwig M, Lütjohann D.et al Neurotoxicity of 24‐hydroxycholesterol, an important cholesterol elimination product of the brain, may be prevented by vitamin E and estradiol‐17β. J Neural Transm 2001108475–488. [DOI] [PubMed] [Google Scholar]
- 3.Papassotiropoulos A, Streffer J R, Tsolaki M.et al Increased brain β‐amyloid load, phosphorylated tau, and risk of Alzheimer disease associated with an intronic CYP46 polymorphism. Arch Neurol 20036029–35. [DOI] [PubMed] [Google Scholar]
- 4.Lehtovirta M, Soininen H, Helisalmi S.et al Clinical and neuropsychological characteristics in familial and sporadic Alzheimer's disease: relation to apolipoprotein E polymorphism. Neurology 199646413–419. [DOI] [PubMed] [Google Scholar]
- 5.Kölsch H, Lütjohann D, Ludwig M.et al Polymorphism in the cholesterol 24S‐hydroxylase gene is associated with Alzheimer's disease. Mol Psychiatry 20027899–902. [DOI] [PubMed] [Google Scholar]