Abstract
In a large cohort of 1034 patients with the diagnosis of definite or probable amyotrophic lateral sclerosis (ALS), the association of forced vital capacity (FVC) at baseline with (a) time to progression of 20 points in Appel ALS (AALS) score or (b) tracheostomy free survival was investigated. The median survival of ALS patients with baseline FVC <75% was 2.91 years, compared with 4.08 years for patients with baseline FVC >75% (p<0.001). Patients with baseline FVC <75% progressed more rapidly (taking 8.0 months to progress 20 AALS points) compared with patients with baseline FVC >75% (10.0 months, p<0.001). Moreover, FVC at first examination was identified as a significant predictor of survival and disease progression in both univariate and multivariate Cox regression models, after adjustment for age, sex, site of onset, diagnostic delay, riluzole therapy, and use of bilateral positive airway pressure and percutaneous endoscopic gastrostomy (p<0.001). We conclude that a single FVC value obtained at an initial visit may serve as a clinically meaningful predictor of survival and disease progression in ALS.
Keywords: ALS, outcome predictors, survival, disease progression, forced vital capacity (FVC)
Most ALS deaths are due to a decline in pulmonary function resulting from respiratory muscle weakness,1,2,3 and the extent of respiratory involvement has been reported as a major prognostic factor.4 At present, no single test has been shown to reliably detect early respiratory insufficiency and to correlate with respiratory failure in ALS.5,6 Forced vital capacity (FVC) is one index of respiratory function that may be used to indicate potential respiratory compromise in ALS.7,8,9,10,11 Because FVC assesses inspiratory muscle strength and does not take into account the important prognostic role of expiratory muscles, additional measures, such as sniff nasal pressure, maximum inspiratory pressure, and maximum expiratory pressure, may be also needed to assess the global respiratory function of ALS patients. The accuracy of FVC measurement is highly dependent on the subject's effort and cooperation and on the coaching of the evaluator. Consequently, there is a clear need for continuous training of evaluators to reduce variability in measurements. However, despite these limitations, FVC measurement has been established as a recommended test for clinical trials and an important standard of ALS management.8
The prognostic value of respiratory function measures for survival in ALS has previously been shown in several randomised clinical trials.1,12,13,14,15 Unfortunately, the restrictive inclusion criteria and short follow up time periods may render those data inapplicable to the general ALS population.16 Studies of clinic based populations have suggested a correlation between tracheostomy free survival and the rate of decline in pulmonary function, defined as either the slope of a pulmonary score 4,9 or the rate of FVC decline.17,18 However, other investigators have failed to confirm this or have found no prognostic value after correcting the data for relevant demographic or clinically meaningful covariates.16
METHODS
Patients
Data from 1034 patients with the diagnosis of definite or probable ALS according to El Escorial/Airlie House criteria19,20 who have been regularly followed at our MDA‐ALS clinic over the last 21 years were reviewed in this study.
FVC measurement
FVC was performed as recommended for ALS clinical trials using standard techniques in the sitting position and expressed as a percentage of the expected value.8 FVC was examined at entry and at each follow up visit (in general, every 3 months).
Outcome measurement
Survival and time to progression of 20 points in Appel ALS (AALS) score were selected as outcome parameters. Survival was defined as the number of months from symptom onset to death or tracheostomy. As an alternative to viewing disease progression as a mean rate of change in the given score or the score change from baseline to the study endpoint, we considered time to 20 point increase in AALS total score from baseline examination as failure time, which was determined from patient examination scores. A 20 point change was chosen because it is indicative of a clinically evident change in a patient's clinical status and ability to perform activities of daily living.4 Moreover, the average time to 20 point change was <1 year for both baseline FVC groups, creating an endpoint that is likely to occur during the time frame of many clinical trials.21 Details of the AALS score, which is routinely used in our clinic, have been published previously.4,21,22,23 Patients who remained alive without tracheostomy and patients who never exceeded a 20 point increase in AALS score over the study period were censored at the time of the last known follow up.
Data analysis
The effects of individual prognostic factors on survival and on disease progression were assessed using the Kaplan‐Meier life table methods. Log rank test was used to assess equality of outcome functions. The prognostic value of first FVC measurement was expressed in terms of hazard ratio (HR). Adjusted HRs were estimated by multivariate analyses using the Cox proportional hazards regression model. A p value of <0.05 was considered significant. All statistical analyses were performed using SPSS software (version 11.5.1; SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics
The mean (SD) age at disease onset in our patient population was 54.1 (13.2) years (57.6 for men and 52.4 for women). The male to female ratio was 1.9:1. Time from first symptom to the first examination (FS to FE) was 16.3 (12.3) months. In total, 172 patients (16.6%) exhibited primary bulbar symptoms; 576 (55.7%) of the 1034 analysed patients died (n = 477) or had tracheostomy (n = 99). The median tracheostomy free survival time was 3.45 years (95% confidence interval (CI) 3.27 to 3.74, mean 4.32 years). The median time to 20 AALS point progression was 9.0 months (95% CI 9.00 to 9.00).
Prognostic value of baseline FVC
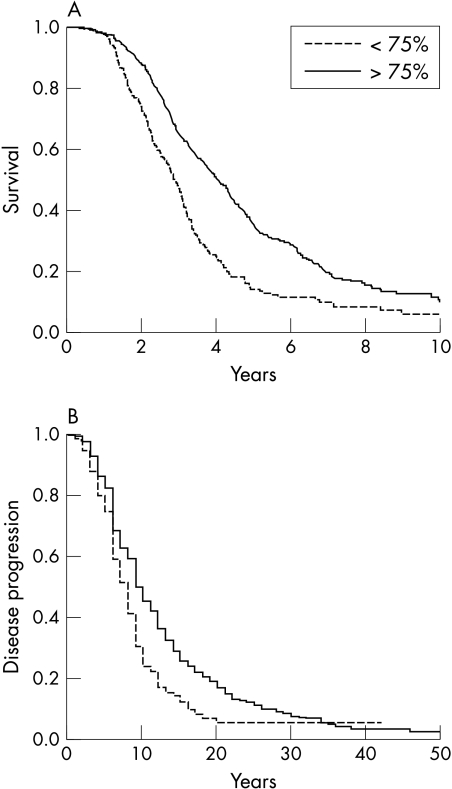
We investigated the association of the FVC at baseline with (a) tracheostomy free survival or (b) time to 20 point AALS score progression from baseline examination for two subgroups of patients with baseline FVC value above (n = 690) and below 75% (n = 344). This FVC value was not only clinically relevant (mean (SD) baseline FVC 82.9 (20.9)%) but was also the optimum cutoff point, resulting in stratification into two large populations that were significantly different in terms of survival and disease progression. The median survival of ALS patients with baseline FVC <75% was 2.91 years, compared with 4.08 years for patients with baseline FVC >75% (log rank p<0.001) (fig 1A). In addition, patients with baseline FVC value <75% progressed more rapidly (8.0 months to 20 AALS points progression) compared with patients with baseline FVC >75% (10.0 months, p<0.001) (fig 1B). Univariate analyses indicated that lower FVC at baseline was associated with shorter survival (HR 1.68; 95% CI 1.22 to 2.00; p<0.001) and with more rapid disease progression (1.57; 1.29 to 1.91; p<0.001).
Figure 1 Kaplan‐Meier plots of (A) survival and (B) time to 20 point progression probabilities according to the first examination FVC value. Patient population was divided into two groups: FVC >75% and FVC <75%.
Given the importance of well established outcome predictors, the hazard ratio and 95% CI were adjusted for age, sex, and site of symptom onset. In this multivariate model, baseline FVC remained significantly associated with both survival (HR 1.73; 95% CI 1.45 to 2.05; p<0.001) and disease progression (1.49; 1.22 to 1.81; p<0.001). We also analysed the influence of other potential confounding variables on our results. Riluzole (50 mg twice daily) was given to 36% of patients with FVC <75% and to 44% of patients with baseline FVC >75%. Non‐invasive ventilation (NIV) was performed in 10.8% of patients in the group with first FVC <75% and 13.2% of patients in the group with FVC >75%. During the disease course, 32% of patients in the FVC <75% group and 24% of those with FVC >75% underwent percutaneous endoscopic gastrostomy (PEG). To explore whether the predictive value of baseline FVC is independent of those factors, we included riluzole, NIV, and PEG use (as time independent variables: “ever” versus “never”) in the same multivariate Cox regression model along with age, sex, site of onset, and FS to FE time. In this final model, baseline FVC remained significantly and independently associated with both survival (HR 2.07, p<0.001) and time to 20 point progression (HR 1.56, p<0.001) (table 1). Of note, when dealing with the use of riluzole, NIV, and PEG, our database only allowed an ever versus never analysis and was not designed to analyse the timing of the intervention or to address the compliance issue for those therapies.
Table 1 Multivariate analyses, with all factors analysed in the same Cox models.
| Category | Survival, years (HR (95% CI)) | p | Disease progression, years (HR (95% CI)) | p | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline FVC <75% | 2.07 (1.73 to 2.48) | <0.001 | 1.56 (1.28 to 1.90) | <0.001 | ||||
| Age at onset, years | 1.04 (1.03 to 1.05) | <0.001 | 1.01 (0.99 to 1.01) | 0.01 | ||||
| Limb vs. bulbar onset | 1.09 (0.87 to 1.37) | 0.4 | 1.34 (1.07 to 1.68) | 0.009 | ||||
| FS to FE time, months | 0.95 (0.94 to 0.96) | <0.001 | 0.98 (0.97 to 0.98) | <0.001 | ||||
| Women versus men | 1.10 (0.92 to 1.33) | 0.3 | 1.20 (0.99 to 1.43) | 0.05 | ||||
| Riluzole (ever versus never) | 1.14 (0.94 to 1.38) | 0.2 | 1.12 (0.95 to 1.32) | 0.2 | ||||
| NIV (ever versus never) | 0.77 (0.62 to 0.95) | 0.02 | ND | ND | ||||
| PEG (ever versus never) | 0.74 (0.62 to 0.88) | 0.001 | ND | ND |
In most patients who received riluzole, therapy was initiated at the time of the first examination and diagnosis, prior to a documented 20 point progression. In contrast, NIV initiation and PEG placement were generally performed in patients who had reached an advanced degree of disability. Thus, the Cox model for disease progression did not include NIV and PEG use.
DISCUSSION
Our data demonstrate that baseline FVC, easily obtained early in the disease course, and typically at the first clinical visit, may serve as an early predictor of survival and disease progression in an ALS clinic population. Because FVC directly assesses pulmonary function, the decline of which is the most common cause of death or chronic respiratory support in ALS patients,1,2 our findings were not surprising. However, to our knowledge, no previous positive data about the predictive value of FVC on disease progression in ALS have been published, and other investigators were unable to confirm a correlation between ALS functional rating scale and FVC.6
The interpretation of our data has several potential limitations that should be recognised. Firstly, entry of patients into our database was non‐random, because nearly all of the patients were referred by neurologists or primary care physicians. Secondly, because of access to optimum medical treatment at an academic institution, patients seen at a referral centre may have a relatively better prognosis.24 Thirdly, during the 20 year observation period in this study, different diagnostic criteria have been in use. However, in our experience, the clinical features of patients diagnosed as “typical ALS” (this term was in use in our database prior to publication of El Escorial criteria in 1994) correspond very closely to the features of patients diagnosed as definite or probable ALS after 1994.19
Our findings may have several consequences for patient management and the planning of symptomatic care in ALS. Moreover, the results of this study also provide a tool for baseline stratification for clinical trials. It is well known that matching patients for established demographic and clinical parameters, such as age, sex, site of symptom onset, and diagnostic delay, is particularly important in planning clinical trials. Because pulmonary function is a critical factor for survival and disease progression, it should be strongly considered during the planning of clinical trials.
ACKNOWLEDGEMENTS
We wish to thank the patients and the members of the Muscular Dystrophy Association ALS Clinic team at Methodist Neurological Institute and Baylor College of Medicine in Houston, Texas for their contributions to gathering the database information. This work was supported by the MDA and the Houston Endowment. A Czaplinski was the recipient of Sheila Essey Award Fellowship and was also supported by Swiss National Science Foundation and Fonds zur Foerderung des Akademischen Nachwuchses University of Basel, Switzerland. We thank A Schoetzau for his thoughtful statistical advice and comments.
Abbreviations
AALS - Appel ALS
ALS - amyotrophic lateral sclerosis
FE - first examination
FS - first symptom
FVC - forced vital capacity
NIV - non‐invasive ventilation
PEG - percutaneous endoscopic gastrostomy
Footnotes
Competing interests: none
References
- 1.Traynor B J, Zhang H, Shefner J M.et al Functional outcome measures as clinical trial endpoints in ALS. Neurology 2004631933–1935. [DOI] [PubMed] [Google Scholar]
- 2.Caroscio J T, Mulvihill M N, Sterling R.et al Amyotrophic lateral sclerosis: its natural history. Neurol Clin 198751–8. [PubMed] [Google Scholar]
- 3.Kleopa K A, Sherman M, Neal B.et al Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci 199916482–88. [DOI] [PubMed] [Google Scholar]
- 4.Haverkamp L J, Appel V, Appel S H. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain 1995118707–719. [DOI] [PubMed] [Google Scholar]
- 5.Miller R G, Munsat T L, Swash M.et al Consensus guidelines for the design and implementation of clinical trials in ALS. J Neurol Sci 19991692–12. [DOI] [PubMed] [Google Scholar]
- 6.Jackson C E, Rosenfeld J, Moore D H.et al A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. J Neurol Sci 200119175–78. [DOI] [PubMed] [Google Scholar]
- 7.Schiffman P L, Belsh J M. Pulmonary function at diagnosis of amyotrophic lateral sclerosis. Chest 1993103508–513. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann J R, Andres P, Mendoza M.et al Guidelines for the use and performance of quantitative outcome measures in ALS clinical trials. J Neurol Sci 199714797–111. [DOI] [PubMed] [Google Scholar]
- 9.Ringel S P, Murphy J R, Alderson M K.et al The natural history of amyotrophic lateral sclerosis. Neurology 1993431316–1322. [DOI] [PubMed] [Google Scholar]
- 10.Gay P C, Westbrook P R, Daube J R.et al Effets of alterations in pulmonary function and sleep variables on survival in patients with amyotrophic lateral sclerosis. Mayo Clin Proc 199166686–694. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan L M, Hollander D. Respiratory dysfunction in amyotrophic lateral sclerosis. Clin Chest Med 199415675–681. [PubMed] [Google Scholar]
- 12.Cudkowicz M E, Shefner J M, Schoenfeld D A.et al A randomized, placebo‐controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology 200361456–464. [DOI] [PubMed] [Google Scholar]
- 13.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med 1994330585–591. [DOI] [PubMed] [Google Scholar]
- 14.Stambler N, Charatan M, Cedarbaum J M. Prognostic indicators of survival in ALS. Neurology 19985066–72. [DOI] [PubMed] [Google Scholar]
- 15.Moore D H, Miller R G, WALS Study Group, ALS CARE Study Group ALSRFS as a measure of disease progression and survival. The Amyotroph Lateral Scler Other Motor Neuron Disord 20034(suppl 1)42 [Google Scholar]
- 16.Kaufmann P, Levy G, Thompson J L.et al The ALSFRSr predicts survival time in an ALS clinic population. Neurology 20056438–43. [DOI] [PubMed] [Google Scholar]
- 17.Chio A, Mora G, Leone M.et al Piemonte and Valle d'Aosta Register for ALS (PARALS). Early symptom progression rate is related to ALS outcome: a prospective population‐based study. Neurology 20025999–103. [DOI] [PubMed] [Google Scholar]
- 18.Magnus T, Beck M, Giess R.et al Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve 200225709–714. [DOI] [PubMed] [Google Scholar]
- 19.Brooks B R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 199412496–107. [DOI] [PubMed] [Google Scholar]
- 20.Traynor B J, Codd M B, Corr B.et al Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: A population‐based study. Arch Neurol 2000571171–1176. [DOI] [PubMed] [Google Scholar]
- 21.Lange D J, Murphy P L, Diamond B.et al Selegiline is ineffective in a collaborative double‐blind, placebo‐controlled trial for treatment of amyotrophic lateral sclerosis. Arch Neurol 19985593–96. [DOI] [PubMed] [Google Scholar]
- 22.Yen A A, Simpson E, Haverkamp L J.et al AALS. Amyotroph Lateral Scler Other Motor Neuron Disord 20045(suppl 1)S1–S5. [DOI] [PubMed] [Google Scholar]
- 23.Appel V, Stewart S S, Smith G.et al A rating scale for amyotrophic lateral sclerosis: Description and preliminary experience. Ann Neurol 198722328–333. [DOI] [PubMed] [Google Scholar]
- 24.Lee J R, Annegers J F, Appel S H. Prognosis of amyotrophic lateral sclerosis and the effect of referral selection. J Neurol Sci 1995132207–215. [DOI] [PubMed] [Google Scholar]