Abstract
Cardiac sympathetic dysfunction was investigated using 123I‐metaiodobenzylguanidine (MIBG) myocardial scintigraphy in 20 patients with drug induced parkinsonism (DIP). The mean heart to mediastinum ratio was significantly greater in patients with DIP than in those with Parkinson's disease (mean (SD): 2.07 (0.39) v 1.28 (0.15), p<0.001). MIBG uptake was not different between the DIP patients and controls. Two DIP patients whose MIBG uptake was significantly reduced showed persistent parkinsonism and responded dramatically to levodopa.
Keywords: cardiac MIBG, drug induced parkinsonism
Drug induced parkinsonism (DIP) comprises 10–30% of all cases of parkinsonism.1,2,3 The clinical manifestations of DIP resemble those of Parkinson's disease, including high frequency of tremor and asymmetrical signs.4,5,6,7,8 After discontinuation of the offending drug, most patients with DIP are free of parkinsonism within a few weeks.9 However, DIP is not always reversible. It has been reported that Parkinson's disease can develop after apparent recovery from DIP, and that it can persist and eventually worsen after discontinuation of the offending drug.5,9,10
There is little published information about functional imaging in patients with DIP. Using F‐dopa PET, only Burn and Brooks9 systemically reported the integrity of nigrostriatal projections in patients with DIP, showing that 31% of cases had underlying nigrostriatal dysfunction. 123I‐metaiodobenzylguanidine (MIBG) is a noradrenaline (norepinephrine) analogue that is taken up and stored in the sympathetic nerve endings.11 It was shown recently that cardiac MIBG uptake is significantly reduced in patients with Parkinson's disease.12 In the present study, we investigated cardiac sympathetic dysfunction in patients with DIP, using MIBG myocardial scintigraphy.
Methods
We prospectively enrolled 20 patients with DIP, 32 with Parkinson's disease, and 20 healthy controls. DIP was defined by the following criteria:
the presence of least two of four cardinal signs (tremor, rigidity, bradykinesia, and impaired postural reflexes);
the absence of a personal history of extrapyramidal disorders before treatment with the offending drug;
onset of symptoms in the course of treatment with the offending drug.
Parkinson's disease was diagnosed according to United Kingdom Parkinson's disease Society Brain Bank clinical diagnosis criteria.13 All patients with DIP were followed up closely for nine months after withdrawal of the offending drug. The clinical stages of parkinsonism were assessed at the beginning of evaluation and nine months after withdrawal of the offending drug according to the classification of Hoehn and Yahr.14 None of the patients had a history of neuropathy or previous relevant cardiac disease, and routine chest radiography and electrocardiography showed no abnormalities. As a control group, age matched subjects with no history of neurological or heart disease were enrolled.
The 123I‐MIBG (111 mBq) was injected intravenously into each subject. Three hours later, the image of cardiac uptake was taken using dual head gamma camera system (MultiSPECT III, Siemens Medical Systems, Malvern, Pennsylvania, USA). The region of interest was placed on the whole heart and mediastinum of the frontal image. The ratio of 123I‐MIBG uptake in regions of interest of the heart to that in mediastinum (H/M ratio) was calculated. Informed consent was obtained from all subjects.
The Mann–Whitney U test was used for examining the intentional difference between two groups using commercially available software package (SPSS, version 10.0). Probability (p) values less than 0.05 were considered significant.
Results
There was no difference in age among the patients with DIP (mean (SD), 65.8 (7.9) years), patients with Parkinson's disease (64.6 (11.4) years), and the controls (63.7 (10.1) years). The female to male ratio tended be higher in patients with DIP than in the controls (75% v 45%, p = 0.053). The mean duration of parkinsonism in patients with Parkinson's disease and DIP was 3.7 (2.6) years and 8 (1) months, respectively. The details of DIP patients and their offending drugs are summarised in table 1.
Table1 The demographic features in patients with dug induced parkinsonism and their offending drugs.
| Patient | Age/sex | Offending drug (duration, month) | Initial H&Y | Follow up H&Y | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 65/F | Levosulpiride (6) | 2.5 | 0 | ||||
| 2 | 69/F | Levosulpiride (10) | 2 | 0 | ||||
| 3 | 71/F | Levosulpiride (5) | 2.5 | 0 | ||||
| 4 | 51/M | Levosulpiride (18) | 2 | 0 | ||||
| 5 | 70/F | Levosulpiride (20) | 3 | 0 | ||||
| 6 | 67/F | Levosulpiride (46) | 2.5 | 0 | ||||
| 7 | 72/M | Levosulpiride (4) | 2.5 | 2.5 | ||||
| 8 | 76/F | Levosulpiride (6) | 3 | 1 | ||||
| 9 | 67/F | Levosulpiride (2) | 2 | 0 | ||||
| 10 | 66/F | Levosulpiride (10) | 3 | 0 | ||||
| 11 | 64/F | Metoclopramide (5) | 2.5 | 0 | ||||
| 12 | 68/M | Perphenazine (120) | 3 | 1 | ||||
| 13 | 73/M | Perphenazine (84) | 2.5 | 0 | ||||
| 14 | 60/F | Perphenazine (26) | 2.5 | 0 | ||||
| 15 | 41/F | Risperidone (5) | 5 | 1 | ||||
| 16 | 68/F | Risperidone (2) | 2.5 | 2 | ||||
| 17 | 65/M | Risperidone (5) | 3 | 0 | ||||
| 18 | 64/F | Chlorpromazine (20) | 4 | 1 | ||||
| 19 | 72/F | Haloperidol (5) | 2.5 | 0 | ||||
| 20 | 66/F | Cilnidipine (12) | 2.5 | 0 |
H&Y, Hoehn and Yahr stage.
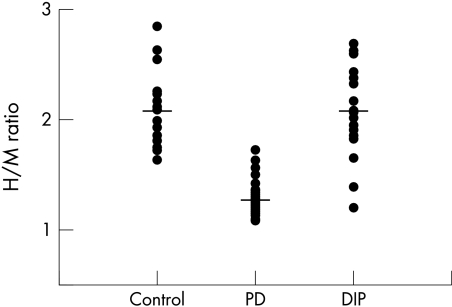
The mean H/M ratio was significantly greater in patients with DIP than in those with Parkinson's disease (2.07 (0.39) v 1.28 (0.15), p<0.001). However, the mean H/M ratio for the DIP group was not significantly different from that in the control group (2.06 (0.33)). Figure 1 shows a scatterplot of the individual cardiac MIBG uptakes in 20 patients with DIP, compared with the Parkinson's disease patients and the controls. Eighteen of 20 patients with DIP had an H/M ratio that lay within 2 SD of the normal mean, but two lay more than 2 SD below the normal mean and fell within the range of the Parkinson patients.
Figure 1 Scatter diagram of individual heart to mediastinum ratio (H/M ratio) of 123I‐MIBG uptake in patients with drug induced parkinsonism (DIP), compared with patients with Parkinson's disease (PD) and the controls. Horizontal lines indicate the mean values.
Eighteen patients with DIP whose MIBG uptake lay within the normal range showed dramatic improvement or complete resolution of parkinsonism on clinical follow up after withdrawal of the offending drug, whereas two DIP patients (numbers 7 and 16) whose MIBG uptake was significantly reduced had persistent parkinsonism. Both of these patients responded well to treatment with levodopa (54% and 76% improvement on motor UPDRS, respectively). There were no clinical characteristics that distinguished those patients who subsequently improved from their parkinsonism from those who did not.
Discussion
Neuroleptics, calcium channel blocker, and dopamine depleting agents all have effects on dopaminergic receptors that may result in DIP. There may be several possible outcomes15: full and long lasting recovery of DIP with no subsequent development of Parkinson's disease (that is, DIP is unrelated to Parkinson's disease); persistence and eventual worsening of parkinsonism after discontinuation of the offending drug (DIP unmasks Parkinson's disease); full remission of DIP after withdrawal of the offending drug with subsequent reappearance of parkinsonism (DIP antedates Parkinson's disease).
It is difficult to identify preclinical Parkinson's disease in patients with DIP because the reversibility of parkinsonian features does not rule out preclinical Parkinson's disease, nor does a clinically rapid resolution of parkinsonism preclude the later development of Parkinson's disease. Moreover, a patient with DIP that is unrelated to Parkinson's disease cannot be distinguished from a DIP patient who has subclinical Parkinson's disease.15 However, as the prognosis and treatment strategies for DIP and Parkinson's disease are different, it is important to be able to recognise subclinical Parkinson's disease.
In an F‐dopa PET study, Burn and Brooks identified nigral dysfunction in DIP patients.9 They suggested that subclinical nigral pathology was a relatively common occurrence in DIP patients, and that affected individuals had continued or worsening parkinsonism, in contrast to those with normal putamenal tracer uptake, who showed subsequent improvement of parkinsonism. In our study, most of the patients with DIP (90%) had cardiac MIBG uptake within the normal range, suggesting that they did not have the sympathetic dysfunction seen in Parkinson's disease, and that their DIP may be unrelated to Parkinson's disease. However, two patients with DIP (10% of the DIP group) showed markedly low cardiac MIBG uptake, falling into the range of Parkinson's disease patients. As in the study by Burns and Brooks,9 the two patients with low cardiac MIBG uptake had persistent parkinsonism after the offending drug was withdrawn, and both patients responded dramatically to levodopa. Thus it is speculated that in these patients the offending drugs may have aggravated the potential dopaminergic defect and thus unmasked the clinical manifestation of Parkinson's disease.
Various drugs have been reported to influence cardiac MIBG uptake.11 Haloperidol decreases MIBG uptake at very highly concentration, however—which is far above that achieved therapeutically.16 Cilnidipine, third generation dihydropyridine based calcium antagonist, is reported to enhance cardiac MIBG uptake and this would lead to falsely raised values; however, this effect is likely to be minimal because the increase in the H/M ratio after cilnidipine treatment is very slight (∼0.1).17 Chlorpromazine and perphenazine might be expected to interfere with cardiac MIBG uptake11; however, there was no direct evidence of this. Finally, there are no reports of drug interactions between levosulpiride, metoclopramide, or risperidone and MIBG. Thus we believe that in present study the influence of the offending drugs on the MIBG uptake was likely to have been trivial. Because the number of offending drugs was small, however, we were unable to show in this study whether DIP patients without preclinical Parkinson's disease all have a normal MIBG uptake or not.
Compared with F‐dopa PET, 123I‐MIBG myocardial scintigraphy is more widely available, requires less sophisticated equipment and analytic procedures, and is cheaper. Our study suggests that cardiac MIBG uptake is in the normal range in a majority of patients with DIP, suggesting that in these patients the condition is unrelated to Parkinson's disease. However, cardiac MIBG uptake can be abnormal in some patients with DIP and in these cases the disorder is clinically similar to Parkinson's disease.
A recent study suggests that low MIBG uptake does not necessarily indicate Parkinson's disease.18 Thus further studies using fluorodopa PET or dopamine transporter imaging need to be done to identify whether DIP patients with reduced cardiac MIBG uptake may have nigral dopaminergic degeneration.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (0412‐DB00‐010‐0007).
Abbreviations
DIP - drug induced parkinsonism
H/M ratio - heart to mediastinum ratio
MIBG - 123I‐metaiodobenzylguanidine
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Competing interests: none declared
References
- 1.Bower J H, Maraganore D M, McDonnell S K.et al Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology 1999521214–1220. [DOI] [PubMed] [Google Scholar]
- 2.Benito‐Leon J, Bermejo‐Pareja F, Morales‐Gonzalez J M.et al Neurological Disorders in Central Spain (NEDICES) Study Group. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology 200462734–741. [DOI] [PubMed] [Google Scholar]
- 3.de Lau L M, Giesbergen P C, de Rijk M C.et al Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 2004631240–1244. [DOI] [PubMed] [Google Scholar]
- 4.Ayd F J. A survey of drug‐induced extrapyramidal reactions. JAMA 19611751054–1060. [DOI] [PubMed] [Google Scholar]
- 5.Hardie R J, Lees A J. Neuroleptic‐induced Parkinson's syndrome: clinical features and results of treatment with levodopa. J Neurol Neurosurg Psychiatry 198851850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller L G, Jankovic J. Metoclopramide‐induced movement disorders. Arch Intern Med 19891492486–2492. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez‐Jimenez F J, Garcia‐Ruiz P J, Molina J A. Drug‐induced movement disorders. Drug Saf 19973180–204. [DOI] [PubMed] [Google Scholar]
- 8.Rocca W A, Bower J H, McDonnell S K.et al Time trends in the incidence of parkinsonism in Olmsted County, Minnesota. Neurology 200157462–467. [DOI] [PubMed] [Google Scholar]
- 9.Burn D J, Brooks D J. Nigral dysfunction in drug‐induced parkinsonism: an 18F‐dopa PET study. Neurology 199343552–556. [DOI] [PubMed] [Google Scholar]
- 10.Stephen P J, Williamson J. Drug‐induced parkinsonism in the elderly. Lancet 198421082–1083. [DOI] [PubMed] [Google Scholar]
- 11.Solanki K K, Bomanji J, Moyes J.et al A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta‐iodobenzylguanidine (MIBG). Nucl Med Commun 199213513–521. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein D S. Dysautonomia in Parkinson's disease: neurocardiological abnormalities. Lancet Neurol 20032669–676. [DOI] [PubMed] [Google Scholar]
- 13.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinsonism – a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoehn M M, Yahr M D. Parkinsonism: onset, progression and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 15.Tolosa E, Coelho M, Gallardo M. DAT imaging in drug‐induced and psychogenic parkinsonism. Mov Disord 200318S28–S33. [DOI] [PubMed] [Google Scholar]
- 16.Mayer S, Karanikas G, Rodrigues M.et al Influence of drugs on myocardial iodine‐123 metaiodobenzylguanidine uptake in rabbit myocardium. Eur J Nucl Med 200027340–345. [DOI] [PubMed] [Google Scholar]
- 17.Sakata K, Shirotani M, Yoshida H.et al Effects of amlodipine and cilnidipine on cardiac sympathetic nervous system and neurohormonal status in essential hypertension. Hypertension 1999331447–1452. [DOI] [PubMed] [Google Scholar]
- 18.Nagayama H, Hamamoto M, Ueda M.et al Reliability of MIBG myocardial scintigraphy in the diagnosis of Parkinson's disease. J Neurol Neurosurg Psychiatry 200576249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]