Abstract
Background
Drawing, and the clock drawing task in particular, is widely used as a diagnostic tool in the study of hemispatial neglect. It is generally assumed that the errors in graphic production, such as the misplacement of numbers, reflect a visuospatial deficit, and that drawing production itself (for example, producing the circle) is unimpaired.
Objectives
To test this assumption by examining whether the production of simple circles is affected by neglect.
Methods
16 right hemisphere stroke patients copied circles of various sizes and their drawings were measured for size accuracy.
Results
Patients with more severe neglect produced greater scaling errors, consistently drawing the circle smaller than the original. Errors were not in the horizontal axis alone—shrinkage occurred equally in both height and width axes.
Conclusions
Neglect can co‐occur with constructional difficulties that serve to exacerbate the symptoms presented. This should be taken into account in the assessment of even apparently simple drawing tasks.
Keywords: hemispatial neglect, clock drawing
Hemispatial neglect is a deficit in the processing of information for a portion of space, most frequently the left side.1 Drawing tasks are ubiquitously used in the diagnosis of neglect, as patients often fail to represent the leftward portion of individual objects, or the left half of a multielement scene.2 A pervasive assumption is that drawing errors reflect the visuospatial deficit, and that graphic production itself is unimpaired. We address this issue in relation to the clock drawing test (CDT), a particularly well used method in neglect assessment (that is, it forms part of the behavioural inattention test (BIT)).3 Individuals are typically asked to draw a clock with the hands set at a specified time. Neglect patients are often found to omit the leftward numbers, or to place all of the numbers in the right hand side of the clock face.4 There are many scoring protocols for the CDT,5,6 and all of them primarily concentrate on the placement of the part elements (for example, numbers, hands) within the circle; little attention is paid to the circular perimeter of the clock face when drawings are scored.7,8,9,10 The lack of interest in circle quality may partly reflect the diagnostic significance (and persuasiveness) of poorly placed part elements. Furthermore, the circle does not often appear to be compromised: “One feature of clock drawing that has rarely been mentioned is that the circumference of the clock face is not usually affected. Perhaps the optimal gestalt of the circle precludes the omission of the parts thereof.” (Halligan and Marshall,4 p 15). Yet the arrival at this conclusion is somewhat qualitative—there have been no empirical studies of circle drawing in neglect patients. Here we show that the production of a circle is itself affected by the extent of neglect, which may well set up a patient for failure with the subsequent positioning of the numbers and hands within the face.
Methods
Patients
Sixteen patients, who had all sustained an acute right hemisphere cerebrovascular accident, participated in the study. None suffered from progressive disease, dementia, or psychiatric disorder (see table 1 for patient details). Participants were assessed for symptoms of hemispatial neglect with the conventional subtests of the BIT. Individual BIT scores and the clock drawing component scores appear in table 1.
Table 1 Patient details.
| Patient | Sex | Age (years) | Weeks since CVA | Lesion location | Left visual field deficit | BIT | Clock drawing |
|---|---|---|---|---|---|---|---|
| AS | F | 43 | 4 | R occipito‐parietal, R frontal; old L temporo‐parietal | Unknown | 135 | 0 |
| BB | M | 81 | 4 | R fronto‐parietal; old R pons | Unknown | 96 | 0 |
| JMC | M | 64 | 2 | R basal ganglia | Yes | 61 | 1 |
| WMM | F | 76 | 4 | R posterior frontal | No | 125 | 1 |
| TH | F | 73 | 6 | R temporo‐parietal | No | 129 | 1 |
| MK | F | 76 | 2 | R thalamus | No | 144 | 1 |
| GML | M | 59 | 5 | R centrum semiovale (patchy) | Yes | 141 | 1 |
| LMC | M | 67 | 4 | R thalamus, posterior limb of int. capsule | Unknown | 134 | 1 |
| AC | M | 71 | 8 | R insula, R MCA area; old R occipital | Yes | 144 | 1 |
| IR | F | 83 | 6 | R occipital pole | Yes | 90 | 1 |
| MG | F | 80 | 6 | R parietal | Unknown | 134 | 1 |
| JH | F | 75 | 30 | R putamen and white matter | No | 144 | 1 |
| HML | F | 71 | 6 | R parietal | No | 132 | Not drawn |
| CMP | M | 80 | 6 | R MCA area, head of caudate, ant. lentiform capsule and nucleus | No | 136 | 1 |
| JM | M | 79 | 12 | R occipital, R thalamus; old L thalamus (symptomless) | Yes | 84 | 1 |
| EK | F | 72 | 10 | R MCA area | No | 113 | 1 |
BIT, behavioural inattention test; CVA, cerebrovascular accident; F, female; L, left; M, male; MCA, middle cerebral artery; R, right.
All patients participated with full consent and under approval of University of Glasgow (Department of Psychology) and National Health Service (Southern General Hospital, Glasgow) ethics committees.
Materials
Figures were laser printed in black on plain white A4 (297×210 mm) paper, with a line weight of 2.25 points. Patients drew their response with a pencil. Stimuli were simple line circles, positioned centrally in the upper portion of the page. There were 11 different sizes of circle, with the diameter ranging from 5 to 10 cm in 0.5 cm increments. Each size appeared twice, resulting in a total of 22 trials. The order of trials was fully randomised, with each patient receiving the same order.
Procedure
Stimuli were placed centrally in front of the patient, who was asked to reproduce the figure, as accurately as possible, in the space below. There was no time limit on performance. Once each drawing had been completed, an experimenter marked the starting position on the page, along with the direction in which the circle was drawn.
Results
Scaling accuracy
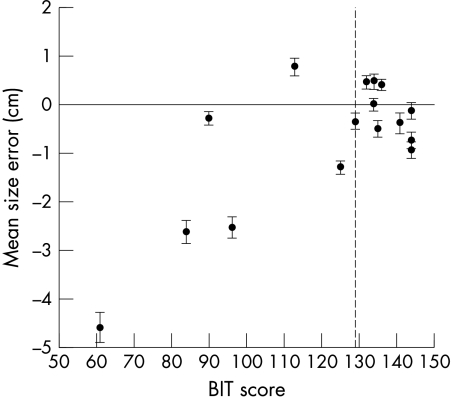
The maximum height (along the vertical axis of the page) and the maximum width of each circle (along the horizontal axis of the page) were recorded. To provide a metric of scaling accuracy, we calculated the difference between the measurements and the actual size of the model stimulus. The size of the model (that is, 5 cm–10 cm) had a significant effect on the scaling accuracy of the drawings: larger model size was associated with greater height error (F(10, 150) = 6.54, p<0.001) and width error (F(10, 150) = 5.65, p<0.001). Overall accuracy was highly associated with severity of neglect. Pearson's correlations found strong associations with BIT scores for height error (r = 0.695, p<0.005) and width error (r = 0.740, p<0.001). For both metrics, patients tended to produce drawings that were smaller than the model—lower BIT scores were associated with greater shrinkage. The relation between BIT and size error (an average of the height and width errors) is illustrated in fig 1.
Figure 1 Relation between BIT scores and mean size error (average of height and width errors): r = 0.742, p<0.001. Error bars are standard error of the mean. The vertical dashed line represents the cut off score for neglect on the BIT: patients with a score of 129/146 or below are classified as showing neglect.
Starting position
The started point for each drawing was classified as being closest to one of eight points, described here as the points of the compass. For 58.2% of trials patients started drawing in the N position; a further 12.8% were at NE, 10.2% at NW, and 11.9% at the W position. These four locations account for 93.2% of responses. On average patients used the same starting position on 81% of trials (range 55–100%). There was no reliable relation between the severity of neglect (as measured by the BIT) and starting point consistency (r = −0.134, p>0.05). There was also no relation between severity of neglect and the percentage of trials in which the starting position was on the right hand side (r = −0.263, p>0.05).
Direction
Most drawings were drawn in the same direction: across all patients, 76.9% of circles were drawn anticlockwise. The size of the model had no effect on the direction that the circle was drawn in (F(10, 150) <1). On average patients drew in the same direction on 95% of trials (range 73–100%). There was no reliable relation between the severity of neglect (as measured by the BIT) and direction consistency (r = −0.269, p>0.05). Patients with neglect were no less likely to make an excursion into the leftward portion of the circle.
Discussion
Neglect patients consistently produced circles that were smaller than the model, and patients who scored lower on the BIT made greater size errors. This deficit in circle drawing occurred even when the task was copying, rather than producing from memory, as occurs in the CDT. Interestingly, the drawings retained equal height/width proportions, suggesting that if there was a distortion of size as part of the neglect syndrome, this was not limited to the horizontal axis as reported previously.11,12 Instead the current results suggest that, for this simple task, the observed distortion was equal for the vertical and horizontal dimensions.
Another possible interpretation is that neglect affects both drawing and visuospatial awareness in separate, but additive, ways. For example, neglect has been associated with a reduction of conscious work space,13 which might lead to a reduced size of drawing. Zelaznik and Lantero14 highlighted the role of vision in the scaling of circles by requiring unimpaired participants to make repetitive circular drawing movements, initially with full vision, and then with vision removed. Circles became smaller, and, as in the current study, there was no difference between height and width measurements. This leads to two important observations: first, something as well practiced as circle drawing still requires visual attention to be successfully executed. Second, patients with deficits of visual attention, as in the neglect syndrome, might as a result encounter difficulty when attempting to produce this figure. In the case of the CDT, the initial part of a clock drawing (that is, the circle) might be produced smaller than would be adequate, and so disrupt the planned placement of the part elements (that is, numbers, hands) within the outline. Of course, this does not fully account for a unilateral placement or omission of parts, but it might contribute to subsequent difficulties such as these.
Finally, The correlation between neglect and circle drawing difficulties reported here may result from both processes being supported by overlapping or anatomically adjacent neural substrates.15,16 This could result in the co‐occurrence of the two separate deficits. Like neglect,17 recent work has suggested that drawing difficulties may arise from a variety of right sided lesions.18
In conclusion, our findings suggest that the interpretation of spatial deficits from the drawings produced by neglect patients can be confounded by deficits in graphic production. Drawing involves the complex interaction of various processes,19,20 which may be compromised in patients with hemispatial neglect and, in turn, affect their performance on drawing tasks. As a result, patients who may appear to present a “pure” perceptual‐attentional neglect deficit on drawing tasks might, in fact, also have a constructional impairment. These difficulties may have an impact on the presentation of the disorder,21,22 and therefore should be taken into account by researchers when designing and analysing neuropsychological tests.
Acknowledgements
We would like to thank Drs Crawford, Muir, and Reeves and Professor Bone for providing patient access, and all the patients for their kind participation. ADS was supported by an ESRC postdoctoral fellowship. SHB is funded by the Royal Society of Edinburgh/Lloyds TSB Foundation.
Abbreviations
BIT - behavioural inattention test
CDT - clock drawing test
Footnotes
Competing interests: none declared
References
- 1.Heilman K M, Watson R T, Valenstein E. Spatial neglect. In: Karnath HO, Milner AD, Vallar G, editors. The cognitive and neural bases of spatial neglect. Oxford: Oxford University Press, 20023–30.
- 2.Halligan P W, Marshall J C. Graphic neglect – more than the sum of the parts. Neuroimage 200114S91–S97. [DOI] [PubMed] [Google Scholar]
- 3.Wilson B A, Cockburn J, Halligan P.Behavioural inattention test. Reading: Thames Valley Test Company, 1987
- 4.Halligan P W, Marshall J C. The history and clinical presentation of neglect. In: Robertson IH, Marshall JC, editors. Unilateral neglect: clinical and experimental studies. Hove: Lawrence Erlbaum Associates, 19933–26.
- 5.Richardson H E, Glass J N. A comparison of scoring protocols on the clock drawing test in relation to ease of use, diagnostic group, and correlations with Mini‐Mental State examination. J Am Geriatr Soc 200250169–173. [DOI] [PubMed] [Google Scholar]
- 6.Tuokko H, Hadjistavropoulos T, Rae S.et al A comparison of alternative approaches to the scoring of clock drawing. Arch Clin Neuropsychol 200215137–148. [PubMed] [Google Scholar]
- 7.Halligan P, Robertson I, Pizzamiglio L.et al The laterality of visual neglect after right hemisphere damage. Neuropsychological Rehabilitation 19911281–301. [Google Scholar]
- 8.Mendez M F, Ala T, Underwood K L. Development of scoring criteria for the clock drawing test in Alzheimer's disease. J Am Geriatr Soc 1992401095–1099. [DOI] [PubMed] [Google Scholar]
- 9.Rouleau I, Salmon D P, Butters N.et al Qualitative and quantitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn 19921870–87. [DOI] [PubMed] [Google Scholar]
- 10.Shulman K I, Shedletsky R, Silver I L. The challenge of time: clock‐drawing and cognitive function in the elderly. Int J Geriatr Psychiatry 19861135–140. [Google Scholar]
- 11.Milner A D, Harvey M. Distortion of size perception in visuospatial neglect. Curr Biol 1995585–89. [DOI] [PubMed] [Google Scholar]
- 12.Harvey M, Olk B, Muir K.et al Eye‐movement patterns do not mediate size distortion effects in hemispatial neglect: looking without seeing. Neuropsychologia 2003411114–1121. [DOI] [PubMed] [Google Scholar]
- 13.Cooney J W, Gazzaniga M S. Neurological disorders and the structure of human consciousness. Trends Cogn Sci 20037161–165. [DOI] [PubMed] [Google Scholar]
- 14.Zelaznik H N, Lantero D. The role of vision in repetitive circle drawing. Acta Psychol 199692105–118. [DOI] [PubMed] [Google Scholar]
- 15.Vallar G. The anatomical basis of spatial hemineglect in humans. In: Robertson IH, Marshall JC, eds. Unilateral neglect: clinical and experimental studies. Hove: Lawrence Erlbaum Associates, 199327–62.
- 16.Mack J L, Levine R N. The basis of visual constructional disability in patients with unilateral cerebral lesions. Cortex 198117515–532. [DOI] [PubMed] [Google Scholar]
- 17.Karnath H O, Fruhmann Berger M, Kuker W.et al The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex 2004141164–1172. [DOI] [PubMed] [Google Scholar]
- 18.Smith A D, Gilchrist I D. Within‐object and between‐object coding deficits in drawing production. Cogn Neuropsychol 200522523–537. [DOI] [PubMed] [Google Scholar]
- 19.Guerin F, Ska B, Belleville S. Cognitive processing of drawing abilities. Brain Cogn 199940464–478. [DOI] [PubMed] [Google Scholar]
- 20.van Sommers P. A system for drawing and drawing‐related neuropsychology. Cogn Neuropsychol 19896117–164. [Google Scholar]
- 21.Wojciulik E, Husain M, Clarke K.et al Spatial working memory deficit in unilateral neglect. Neuropsychologia 200139390–396. [DOI] [PubMed] [Google Scholar]
- 22.Diller L, Riley E. The behavioural management of neglect. In: Robertson IH, Marshall JC, editors. Unilateral neglect: clinical and experimental studies. Hove: Lawrence Erlbaum Associates, 1993293–310.