Abstract
The link between optic neuritis and multiple sclerosis is well established, as is the increased risk of conversion to multiple sclerosis, with lesions seen at presentation on the magnetic resonance imaging (MRI) scan of the brain. One or more asymptomatic lesions were present in 77% of the optic neuritis cohort from London, UK, a higher proportion than that reported in other large cohorts studied elsewhere, where generally lower prevalence rates for multiple sclerosis are also reported. These observations may support the hypothesis that optic neuritis is more likely to be associated with abnormalities on MRI and to be due to multiple sclerosis in geographical regions where multiple sclerosis is more common.
The likelihood of people with clinically isolated optic neuritis developing multiple sclerosis is increased by the presence of brain lesions seen on a magnetic resonance image.1 The proportion of patients with optic neuritis with abnormalities on magnetic resonance imaging (MRI) varies widely between cohorts reported from different regions of the world.2,3,4,5,6 A question arising is whether the frequency of abnormalities on MRI and development of multiple sclerosis in patients with optic neuritis are related to the prevalence of multiple sclerosis7 per se. We explored this idea by examining the frequency of abnormalities on MRI in our optic neuritis cohort from London, UK, and other published studies and the prevalence of multiple sclerosis reported from similar geographical locations.
Methods
Optic neuritis cohort from London
We report on 133 patients with clinically isolated optic neuritis, who attended the Moorfields Eye Hospital, London, UK, and underwent MRI of the brain between 1995 and 2004. All patients were referred by ophthalmologists and reviewed by a single, experienced neuro‐ophthalmologist (GTP). Patients with a history of previous neurological events or signs of abnormality outside the optic nerves were excluded. MRI of the brain was carried out within 3 months of symptom onset. The scans were reviewed by an experienced neuroradiologist (KAM) and the numbers of high signal lesions on both proton density‐weighted and T2‐weighted scans were noted. Scans were reported to be normal if there were no lesions compatible with demyelination.
Optic neuritis cohorts from other regions
Prospective brain MRI studies in five other large optic neuritis cohorts from around the world were identified and the frequency of abnormalities on MRI noted.2,3,4,5,6 Other features of the studies also noted included geographical location and year of study, number of patients, method of referral, recruitment criteria including age range and previous or bilateral optic neuritis or clinical features such as disc pallor, duration of symptoms at the time of MRI and definition of abnormality on MRI and slice thickness.
Regional prevalence estimates of multiple sclerosis
Regional prevalence data on multiple sclerosis were acquired from a recent review by Rosati7 and compared among the countries in which the identified studies on optic neuritis had been conducted: >80 per 100 000 was defined as high prevalence, 30–80 per 100 000 as medium and <30 per 100 000 as low. As there may be variation in regional prevalence of multiple sclerosis in a single country, data from a region close to that of the optic neuritis study were used where possible. An exception was the US, where estimated average prevalence for the country as a whole was used because optic neuritis data were from the Optic Neuritis Treatment Trial (ONTT), which was a multicentre trial on patients from across the country. Using the annually conducted National Health Interview Survey of the US from 1989 to 1994, the national prevalence of multiple sclerosis was estimated at 85 per 100 000.8
Results
London cohort
The patients were aged 16–49 (median 31) years, 92 were women and 41 were men. Two patients had bilateral sequential optic neuritis (2–3 weeks interval between episodes) and 131 had acute unilateral optic neuritis. MRI of the brain was carried out in all cases within 3 months (median 5, range 1–12 weeks) of symptom onset. One or more asymptomatic brain lesions (median 9, range 1–117 lesions) compatible with demyelination were present in 103 (77%) patients.
Optic neuritis cohorts from other areas
Table 1 summarises data from other optic neuritis cohorts from other countries. The frequency of abnormalities on MRI ranged from 14%6 (Japan) to 65%3 (Sweden).
Table 1 Comparing methods and percentage of patients with isolated optic neuritis with baseline abnormalities on magnetic resonance imaging (MRI) and nearest regional prevalence estimate of multiple sclerosis.
| Years of recruitment | MRI slice thickness (mm) | Age range (years) | Maximum time from symptom onset to MRI | Percentage with abnormal MRI | Multiple sclerosis prevalence (per 100 000)7 | |
|---|---|---|---|---|---|---|
| London, England, n = 135 | 1995–2004 | 3 | 16–49 | 3 months | 77 | 104 |
| Stockholm, Sweden, n = 1163 | 1990–1995 | 5 | 12–57 | 24 weeks | 65 | 96 |
| Copenhagen, Denmark, n = 1204 | 1987–1993 | 4 | 12–59 | 4 weeks | 53* | 112 |
| North America, n = 3892 | 1988–1991 | 5 | 18–46 | 8 days | 54† | 858 |
| Barcelona, Spain, n = 1235 | 1995–2001 | 5 | 14–50 | 3 months | 51 | 57 |
| Japan, n = 706 | 1991–1996 | Not specified | 15–55 | 14 days | 14 | 1–4 |
*In the Danish study, abnormal MRI was defined as the presence of at least two lesions, and data for one or more lesions were not given; therefore, these data were not included in fig 1. †Data represent the 90% Optic Neuritis Treatment Trial cohort in whom MRI findings are reported.
MRI of the brain in patients with optic neuritis and regional prevalence of multiple sclerosis
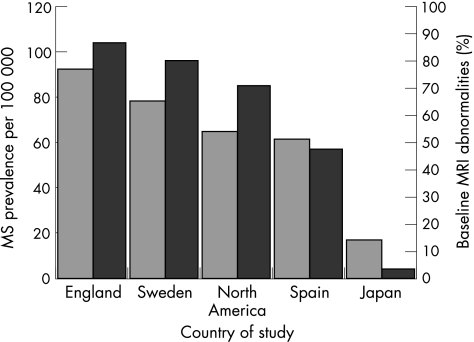
Figure 1 shows the frequency of abnormalities on MRI in patients with optic neuritis, where available, and the corresponding reported regional prevalence for multiple sclerosis.
Figure 1 Variation in the percentage of patients with isolated optic neuritis with baseline abnormalities seen on magnetic resonance imaging (MRI) and the prevalence of multiple sclerosis (MS) in that country.
Discussion
In comparison with rates previously reported in other geographical regions (14–65%),2,3,4,5,6 a higher proportion (77%) of our cohort of patients with optic neuritis from southern England showed, at presentation, abnormalities suggestive of demyelination on MRI of the brain. A high prevalence of multiple sclerosis is well documented in southern England.7 Inspection of the frequency of abnormalities seen on MRI in the optic neuritis cohorts and corresponding regional multiple sclerosis prevalence suggests that the two are broadly related (fig 1), although the apparent link is not entirely consistent and should be interpreted with caution because of the observational nature of the analysis and multiple methodological differences between reported studies.
The Japanese cohort was probably phenotypically different in some visual features from other cohorts—for example, there was a high frequency of poor visual acuity in the Japanese cohort during the acute phase: 61% had visual acuity worse than 6/60 compared with 36% of the ONTT cohort. Multiple sclerosis is much less common in Japan than in Europe or North America. Furthermore, 15–40% of multiple sclerosis cases in Japan are of the opticospinal type, and patients with this multiple sclerosis subtype are reported to have fewer lesions on MRI of the brain than seen in those with conventional multiple sclerosis.9 The overall evidence suggests that optic neuritis in Japan is more often an isolated syndrome with normal MRI and less often due to multiple sclerosis.
The ONTT2 recruited patients from all over the US, limiting comparison of geographical prevalence in studies from smaller, well‐defined catchment areas such as the Scandinavian countries.3,4 However, the ONTT provided a wealth of clinical and MRI data and, like the London cohort, included a multiethnic and immigrant rich population. By contrast, in the Swedish study, all patients were Caucasian and only 4% were of non‐Swedish origin.3 Variation in migration patterns and ethnicity may influence risk and prevalence of multiple sclerosis and add complexity when comparing studies from different geographical regions.
Considering studies from the past 20 years, the incidence of optic neuritis in Japan (1992–3) of 1.6–2 per 100 000 people10 seems to be similar to incidences reported in Sweden (1990–5), 1.46 per 100 00011; Croatia (1985–2001), 1.6 per 100 000; and the UK (1995–6), 1 per 100 000 people.13 A higher incidence was reported from Minnesota with age‐adjusted and sex‐adjusted incidence of 5.1 per 100 000 people14 (1985–91), although epidemiological ascertainment is likely to be particularly high in this well‐defined local region that is served by the Mayo Clinic, Rochester, Minnesota, USA. Earlier studies report incidences <1 per 100 000 people and there may have been improvement in case ascertainment or an increase in incidence with time. A study in Hawaii compared the incidence of optic neuritis in the oriental and the Caucasian populations and found no major difference between the two races (0.7 and 1.1 per 100 000 people, respectively).15 Taken together, these data suggest that the incidence of optic neuritis is relatively similar across regions where the prevalence of multiple sclerosis varies markedly: it is therefore plausible that the frequency of cases with which optic neuritis is due to multiple sclerosis will also vary. As the studies reported did not include other known causes (eg, sarcoidosis, vasculitis, syphilis), it seems likely that in regions with low prevalence of multiple sclerosis—especially when MRI findings are normal—optic neuritis is often a monophasic inflammatory syndrome.
A clinically isolated syndrome study from Barcelona5 reported that people with a brain stem or spinal cord syndrome had a higher frequency (76%) of abnormalities on MRI at presentation and a higher conversion rate to clinically definite multiple sclerosis than did those with optic neuritis (of whom 51% had abnormal MRI at presentation). The Barcelona study also showed that when only those with abnormalities on MRI were compared, no considerable difference in risk for conversion to multiple sclerosis was found between the clinically isolated syndrome subtypes. A medium (around 50%) prevalence of abnormalities on MRI in patients with optic neuritis is concordant with the medium prevalence of multiple sclerosis reported in the same region of Spain.7
The hypothesis that there is a link between proportions of patients with optic neuritis with abnormalities on MRI and corresponding regional prevalence of multiple sclerosis is not proved by the present work, which is observational in nature. Potential biases exist that limit interpretation of the various datasets on optic neuritis cohorts: these include possible referral biases, different inclusion and exclusion criteria (eg, age range, unilateral or bilateral optic neuritis); variations in time from onset of visual symptoms to having MRI; the stringency with which patients with optic neuritis with other neurological features were excluded; differential proportions of long‐term resident versus more frequent migrant populations; use of different MRI scanners, acquisition sequences and slice thicknesses; and variable definitions of abnormalities on MRI. Furthermore, the cited studies on prevalence of multiple sclerosis were conducted at different time periods and may have had their own biases related to identification and diagnosis of patients. Although the observations support a link between frequency of abnormalities on MRI in isolated optic neuritis and regional prevalence of multiple sclerosis, a prospective epidemiological study design that uses a standardised approach to identification and diagnosis of patients would be required for definitive clarification.
Acknowledgements
The NMR Research Unit is supported by a programme grant from the MS Society of Great Britain and Northern Ireland. We thank David MacManus, Ros Gordon and Chris Benton for carrying out the MRI scans.
Abbreviations
MRI - magnetic resonance imaging
ONTT - Optic Neuritis Treatment Trial
Footnotes
Competing interests: DHM received grant support from Biogen Idec, Elan, Schering and GlaxoSmithKline for performance of MRI analyses in clinical trials; honoraria for advisory or consultancy work, lectures and related travel and accommodation expenses from Aventis, Biogen Idec, Bristol Myers Squibb, GlaxoSmithKline, Schering, Serono, UCB Pharma and Wyeth. KF received salary support from Biogen Idec. CMD received salary support from Elan. GTP received travel and accommodation expenses from Alcon. AJT received honoraria for lecturing from Aventis and Schering. The above‐mentioned organisations did not participate in any aspect of the study design, execution, analysis or write up.
References
- 1.Brex P, Ciccarelli O, O'Riordan J.et al A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002346158–164. [DOI] [PubMed] [Google Scholar]
- 2.Beck R W, Cleary P A, Trobe J D.et al The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. N Eng J Med 19933291764–1769. [DOI] [PubMed] [Google Scholar]
- 3.Soderstrom M, Ya‐Ping J, Hillert J.et al Optic neuritis: prognosis for multiple sclerosis from MRI, CSF and HLA findings. Neurology 199850708–714. [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen J L, Madsen H O, Ryder L P.et al HLA typing in acute optic neuritis. Relation to multiple sclerosis and magnetic resonance imaging findings. Arch Neurol 19975476–80. [DOI] [PubMed] [Google Scholar]
- 5.Tintore M, Rovira A, Rio J.et al Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann Neurol 200557210–215. [DOI] [PubMed] [Google Scholar]
- 6.Wakakura M, Minei Higa R, Oono S.et al Baseline features of idiopathic optic neuritis as determined by a multicenter treatment trial in Japan. Jpn J Ophthalmol 199943127–132. [DOI] [PubMed] [Google Scholar]
- 7.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci 200122117–139. [DOI] [PubMed] [Google Scholar]
- 8.Noonan C W, Kathman S T, White M C. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology 200258136–138. [DOI] [PubMed] [Google Scholar]
- 9.Kira J. Multiple sclerosis in the Japanese population. Lancet Neurol 20032117–127. [DOI] [PubMed] [Google Scholar]
- 10.Wakakura M, Ishikawa S, Oono S.et al Incidence of acute idiopathic optic neuritis and its therapy in Japan. Optic Neuritis Treatment Trial Multicenter Cooperative Research Group (ONMRG). Nippon Ganka Gakkai Zasshi 19959993–97. [PubMed] [Google Scholar]
- 11.Jin Y P, de Pedro‐Cuesta J, Soderstrom M.et al Incidence of optic neuritis in Stockholm, Sweden 1990–1995: I. Age, sex. J Neurol Sci 1998159107–114. [DOI] [PubMed] [Google Scholar]
- 12.Bojic L, Ivanisevic M, Sinicic A.et al The incidence of optic neuritis in Split‐Dalmatia county, Croatia. Coll Anthropol 200428343–347. [PubMed] [Google Scholar]
- 13.MacDonald B K, Cockerell O C, Sander J W A S.et al The incidence and lifetime prevalence of neurological disorders in a prospective community‐based study in the UK. Brain 2000123665–676. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez M, Siva A, Cross S A.et al Optic neuritis: a population‐based study in Olmsted County, Minnesota. Neurology 199545244–250. [DOI] [PubMed] [Google Scholar]
- 15.Alter M, Good J, Okihiro M. Optic neuritis in orientals and Caucasians. Neurology 197323631–639. [DOI] [PubMed] [Google Scholar]