Abstract
Background
Horizontal gaze palsy and progressive scoliosis (HGPPS) is caused by mutations of the ROBO3 gene, which encodes a receptor associated with axonal guidance during development. Although there is evidence for uncrossed cuneatal and corticospinal tracts in HGPPS, it is unclear whether other central nervous system pathways are involved.
Objective
To study two patients with HGPPS homozygotic for the ROBO3 E319K mutation using a variety of neurophysiological and neuropsychological tests.
Methods
A battery of neuropsychological tests was applied to assess various cognitive and perceptual functions. The corticospinal, somatosensory and auditory pathways were evaluated using appropriate neurophysiological tests. To access motor pathways to the neck muscles, electromyographic recordings were obtained from the sternocleidomastoideus and splenius capitis muscle during active head rotation.
Results
Both patients performed normally on manual dexterity, complex sensory and visuospatial functions, reading and general intelligence tests. Motor evoked potentials in both patients showed uncrossed corticospinal tracts for the extremities, although in one patient, electromyography indicated pyramidal tract crossing for the neck muscles. Although somatosensory evoked potentials showed uncrossed somatosensory fibres subserving proprioception and light touch, right median nerve somatosensory evoked potential in one patient indicated a partial lemniscal crossing. Sympathetic skin response and blink reflex showed a midline crossing of the spinothalamic and quintothalamic tracts. Brain stem auditory evoked potentials indicated a lack of crossing in the level of the trapezoid body.
Conclusions
Our patients with the ROBO3 E319Κ mutation show normal perceptual and cognitive functions and have both crossed and uncrossed motor, sensory and auditory pathways.
Horizontal gaze palsy and progressive scoliosis (HGPPS) is a rare autosomal recessive disorder characterised by absent conjugate lateral eye movements with preserved vertical gaze and progressive scoliosis.1,2,3,4,5 Although the disorder has been known for over three decades, its pathogenesis remained obscure until Jen et al3 reported that homozygotic mutations in the ROBO3 gene are present in patients with HGPPS. The ROBO3 protein belongs to a family of transmembrane receptors that are expressed in the growth cone of elongating axons, guiding them to contralaterally located target neurones during nervous system development. Ten different mutations (6 missense, 2 frameshift, 1 nonsense, 1 splice site) located in different domains of the encoded protein have been identified and are thought to diminish the function of this receptor,3 with recent imaging and neurophysiological studies providing evidence for the presence of uncrossed motor and sensory pathways in patients with HGPPS.3,4,5,6,7
Although detection of ROBO3 gene mutations has advanced our understanding of the molecular signalling mechanisms that lead to crossing of longitudinal motor and sensory tracts, several issues remain unsolved. Thus, although there is evidence for lack of crossing of the long descending pyramidal tracts and the ascending medial lemniscal projections, it is presently unclear whether other pathways that normally cross the midline are affected and whether the various ROBO3 mutations produce the same anatomical phenotype. Patients with HGPPS seem to face little functional consequences, but it is not known how these patients perform on standardised neuropsychological tests.3
To deal with these issues, we examined two brothers affected by HGPPS, both of whom were homozygotic for the ROBO3 E319K mutation.
Patients and methods
Patient history, physical and neurological examination
We studied two brothers, aged 54 (patient 1) and 45 years (patient 2), who are members of the previously described GD family.3 In addition, we evaluated four of their five siblings. Detailed medical and neurological histories were taken and physical and neurological examinations were carried out using standard methods.
Mutation analysis
Genomic DNA was extracted from whole blood of all consenting family members. All 28 exons and flanking introns of the ROBO3 gene were amplified using primers as previously described.3 Polymerase chain reaction conditions are described in the supplementary data (available online at http://jnnp.bmjjournals.com/supplemental). Automated and bidirectional sequencing of all exons and flanking intronic regions was carried out on the ABI Prism 3100 Genetic Analyser (PE‐Applied Biosystems, Foster City, California, USA).
Magnetic resonance imaging
Magnetic resonance imaging of the brain was carried out using a 1.5‐T scanner (Sonata, Siemens, Germany).
Neuropsychological assessment
Manual dexterity, complex sensory and spatial–visuoperceptual functions, reading and general intelligence were assessed. Tests of sensory function focused on tactile inattention (face–hand test), graphesthesia (adaptation of Rey's test by substituting Greek characters for Latin ones) and stereognosis (version of the manual form perception test).8 Five shapes (circle, triangle, square, diamond and octagon) and five objects of daily use (toothbrush, tennis ball, comb, large cup, and a sweet in a wrapper) were presented to the patients in a random order, with vision occluded. The patient was then asked to point to the object from a selection of drawings representing the 10 objects. The number of objects matched correctly was taken as the stereognosis score. Finger agnosia was examined using the finger localisation test.9,10 In the first part of the test, the patient was asked to identify fingers of their concealed hand touched by the examiner in a random order on a diagram. In the second part, two fingers were touched simultaneously. The patient's ability to perceive and reproduce directional patterns of tactile stimulation was assessed with a modified version of the tactile finger recognition test that is part of the Halstead–Reitan battery.11 In our version the patient was given four sequences of finger taps (two in a left–right and two in a right–left direction) to each hand in the ipsilateral hemispace. The procedure was repeated in the contralateral hemispace (ie, left hand placed to the right of the patient's midline). All the tests listed above were carried out without visual feedback.
Right–left orientation was assessed using 10 simple tasks, such as “Show me your left hand” and “Show me my right eye”.12 Manual dexterity was assessed with the grooved pegboard test.13 The presence of visual neglect was examined with the line bisection test.14 In addition, a line bisection test with sequential stimulus presentation was used.15 The test required the patient to mark the midpoint of 16 horizontal and 16 vertical lines located in the middle of a white sheet of paper. Four line lengths were used (50, 100, 150 and 200 mm, four trials of each length). Two key aspects of reading, phonological decoding and sight‐word recognition, were assessed through timed oral word‐reading tasks. The sight‐word efficiency test consists of 150 high‐frequency words arranged by order of increasing difficulty in four columns on a single sheet, whereas the pseudoword reading efficiency test contains 75 pseudowords constructed by changing two or more sounds in the word stimuli. Finally, general intelligence was assessed using the Greek version of the Wechsler Abbreviated Scales of Intelligence, with normative data adjusted for age and education (Simos et al, 2005, unpublished data).
Motor evoked potentials
Motor evoked potentials were obtained using a magnetic stimulator (MagLite r25, Dantec, Skovlunde, Denmark) with a circular magnetic coil with an outside diameter of 14 cm (MMC 140, Medtronic, Minneapolis, Minnesota, USA). The centre of the coil was placed about 1 cm lateral to the vertex to stimulate the leg area and 2 cm posterior and 2–3 cm lateral to electrode Cz (according to the international 10–20 system) to stimulate the finger area. Surface recording electrodes were placed on the belly tendons of the first dorsal interosseus (FDI) muscle and the anterior tibialis muscle on each side. The responses were recorded with a standardised electrodiagnostic system (Neuropack2, Nihon Kohden, Tokyo, Japan). The same device was also used for the following neurophysiological studies, including the brain stem auditory evoked potentials (BAEPs).
Electromyography
Concentric needle electrodes were inserted into the left sternocleidomastoideus and the right splenius capitis muscles to assess muscle activity during voluntary rightward head rotation.
Median nerve somatosensory evoked potentials
Electrical stimulation with square‐wave pulses was delivered by a bipolar surface electrode at the rate of four 0.3‐ms pulses per second to the left and right median nerves at the wrist. Signal intensity was adjusted to slightly above threshold for producing a thumb twitch. A total of 500 responses was averaged. The recording electrodes (scalp needles) were placed at 2 cm behind C3 (C3′) and C4 (C4′) to record the cortical N20 response. The midfrontal site Fz was used as reference.
Blink reflex
The blink reflex was recorded from the orbicularis oculi muscle (lower eyelid) using surface electrodes referenced to the chin. The supraorbital nerves were stimulated successively using a 0.3‐ms electrical pulse at an intensity of 17 mA. Four traces were recorded and superimposed.
Sympathetic skin response
The sympathetic skin response (SSR) was recorded with surface electrodes placed on the palms and the soles of the feet referenced to the dorsum of the hand and foot, respectively. The left median nerve was stimulated with brief electrical pulses (0.2 ms duration) with an intensity of 30 mA. Four responses were superimposed.
Audiograms
Audiograms were taken using a Clinical Audiometer Orbiter 922, V.2 (Madsen Electronics, Minnetonka, Minnesota, USA) in a special soundproof chamber. Air conduction thresholds were estimated for frequencies ranging between 125 Hz and 8 kHz. The frequency range for bone conduction tests was 250 Hz to 4 kHz.
Tympanometry and acoustic reflex
Tympanograms and stapedius reflexes were obtained using an Impedance Audiometer (Interacoustics AZ 26, Assens, Denmark). Ipsilateral stimulus frequencies were 0.5, 1, 2 or 4 kHz at intensities ranging between 70 and 110 db hearing level. The contralateral stimulus was delivered through a TDH‐39 headphone at the same frequencies, intensity ranging between 70 and 120 db hearing level.
Brain stem auditory evoked potentials
BAEPs were recorded to alternated (rarefaction/condensation) clicks of 0.1‐ms duration presented at an intensity of 80 dB and at a rate of 13 Hz. The filters were set between 50 Hz and 3 kHz. Recordings were carried out bilaterally to monaural stimulation through mastoid needle electrodes referenced to Cz.
Results
Family investigations
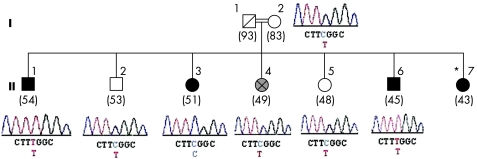
A detailed pedigree of the GD family3 is shown in fig 1. In this family, consanguineous parents (I‐1, I‐2) gave birth to seven children, three of whom are affected by HGPPS. The two brothers evaluated here (II‐1 and II‐6) are homozygotic for the ROBO3 E319K mutation (fig 1). The third affected sibling is a 43‐year‐old sister (II‐7) who presents the typical clinical picture of HGPPS, but no genetic studies have been carried out in her case. In addition, a 49‐year‐old sister (II‐4), affected by a progressive neurological disorder that is clinically distinct from HGPPS, was examined. Since adolescence, she has experienced progressive external ophthalmoplegia (with prominent eyelid ptosis) and progressive myopathy. Scoliosis is not part of her clinical picture. Analysis of her DNA derived from peripheral blood has not shown mitochondrial DNA changes. She has refused a muscle biopsy. Analysis of the entire coding sequence of the ROBO3 showed heterozygosity for the E319K mutation (fig 1).
Figure 1 Family with horizontal gaze palsy and progressive scoliosis (HGPPS). Homozygotic mutation (955G→A) of the ROBO3 gene was found in the two affected brothers (II‐1, II‐6), whereas heterozygotic mutation was present in the 49‐year‐old sister (II‐4), with progressive external ophthalmoplegia and myopathy, as well as in the mother (I‐2) and in two asymptomatic siblings (II‐2, II‐5). The third asymptomatic sibling (II‐3) had two normal alleles. The age at examination (in years) is shown in parentheses below each patient. Filled symbols, patients with HGPPS; grey‐shaded symbol, patient with progressive external ophthalmoplegia and myopathy; *patient with HGPPS not available for DNA testing.
Patients
Both brothers are known to have horizontal gaze palsy since childhood, with scoliosis appearing in adolescence. Although both underwent corrective spine surgery, physical examination showed a marked right curved thoracic scoliosis. Neurological examination showed that both patients did not have conjugate horizontal eye movements with preserved vertical gaze. Abrupt head jerks (preceded by an eye blink) were used for looking left and right. With the head held still, vergence eye movements substituted for attempted lateral gaze. Vertical saccade velocities were in the low normal range and vertical optokinetic nystagmus were preserved. The vestibulo‐ocular reflex was present in the vertical but not in the horizontal plane. A horizontal pendular (at times asymmetrical) nystagmus was present in the primary position. The pupils were equal and reacted normally to light and accommodation consensually. Visual acuity was 20/30 bilaterally in patient 1, and 20/30 on the right and 20/25 on the left eye in patient 2. Funduscopy yielded no abnormal findings. Testing of the corneal reflex produced a normal consensual response. Speech had a nasal quality and a slight hearing loss was noted on the right side in both patients. Muscle power and muscle tone were normal, and tendon reflexes were brisk. Plantar responses were flexor bilaterally. The gait was normal, although both patients had a slight difficulty with tandem walking. In addition, they had difficulty performing alternating movements with the upper and the lower extremities simultaneously. No mirror movements were noted. Examination of the sensory system and coordination was normal.
Magnetic resonance imaging
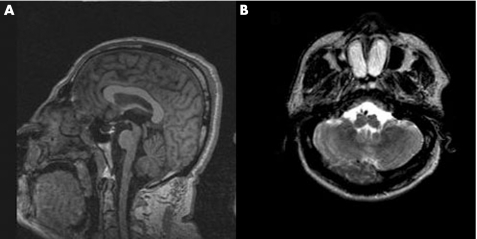
On the midsagittal T1‐weighted view, the corpus callosum and the pons were shown to be normal (fig 2A). On the axial T2‐weighted image at the base of the skull, there was flattening of the anterior surface of the medulla oblongata, with the pyramids appearing hypoplastic (fig 2B).
Figure 2 Normal corpus callosum (A) and flattening of the anterior surface of medulla oblongata in patient 1 (B).
Neuropsychological findings
Both patients are left handed, as indicated by a score of –0.9 and –1.0 on the Edinburgh Handedness Inventory for patient 1 and patient 2, respectively.16 Patient 1 reported early preference for the left hand for most daily activities, but he was forced to use his right hand in childhood for writing. He made no errors on the tactile inattention, graphesthesia, stereognosis, finger agnosia, right–left orientation and finger sequencing tests (tactile finger recognition test), a typical finding among neurologically intact adults. His performance on the line bisection test did not present any omissions. On the single line at a time bisection task, he showed the expected leftward bias (tendency to estimate the middle of the horizontal line slightly to the left of the true midline). The size of leftward midline displacement increased with line length (mean D was 1.8, 2.5, 3.6 and 4 mm for line lengths of 50, 100, 150 and 200 mm, respectively). This trend is typical for neurologically intact people across the lifespan.17 As shown in table 1, the patient performed in the average to low‐average range for his age and education on word reading and general intelligence tests.
Table 1 Performance on standardised intelligence and reading tests.
| Patient | WASI verbal | WASI performance | Sight‐word‐reading efficiency test | Pseudoword‐reading efficiency test |
|---|---|---|---|---|
| 1* | 92 | 88 | 96 | 86 |
| 2† | 87 | 93 | 90 | 85 |
WASI, Wechsler Abbreviated Scales of Intelligence.
*Standard scores based on data for 105 neurologically intact elderly people (age range 55–69 years, mean (SD) 61.5 (3) years, mean years of education 5 (2) years). †Data based on 85 healthy controls (age range 40–50 years, mean (SD) 49 (2) years, mean (SD) years of education 5 (2) years).
Patient 2 showed a similar profile, with the exception of a slight difficulty in discriminating digits 3 and 4 of the left hand (he correctly identified light‐touch stimuli to either digit after five consecutive repetitions). He correctly identified three of eight letters traced on the palm of his left or right hand, and all his errors were visually similar letters to the target (eg, A for Δ, Σ for Z, P for Φ).
Motor evoked potentials
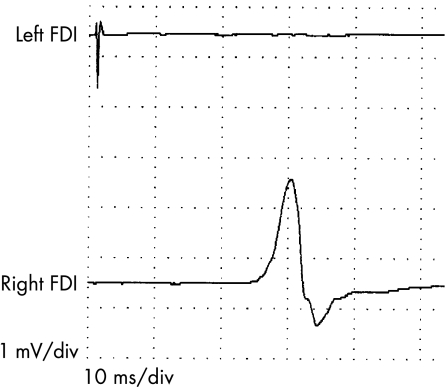
Magnetic pulses delivered at 50–60% of the maximum intensity elicited only ipsilateral responses in the FDI and anterior tibialis muscles, with peak latencies of about 22 and 30 ms, respectively, in both patients (fig 3). Stronger stimuli evoked bilateral responses, as magnetic pulses of high intensity inevitably reach the contralateral cortex. Central motor conduction time to the arms and legs (left and right) was normal in both patients (about 7 ms in the arms and 15 ms in the legs).
Figure 3 Motor evoked potentials (patient 2). Stimuli of 50–60% of the device power were applied to the right motor area. Recordings were obtained simultaneously from the left and right first dorsal interosseous (FDI) muscle. A response was evoked only in the right FDI.
Electromyography
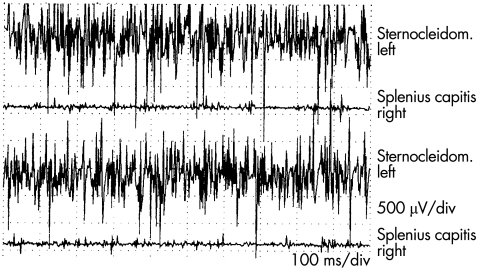
Normally, rightward head rotation is associated with contraction of the left sternocleidomastoideus and the right splenius capitis muscles, and electromyographic (EMG) data were consistent with this pattern in patient 1. In patient 2, voluntary head rotation to the right was associated with normal EMG activity in the left sternocleidomastoideus muscle, but no or little activity was noted in the right splenius capitis (fig 4). On the other hand, contraction in the splenius capitis muscle was noted during neck extension.
Figure 4 Electromyography recordings using concentric needle electrodes during rotation of the head to the right (patient 2). Normal activity was noted in the left sternocleidomastoideus (Sternocleidom.) and no or little activity was noted in the right splenius capitis.
Median nerve somatosensory evoked potentials
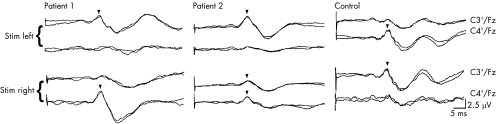
Ipsilateral N20 responses with normal latencies were noted in both patients. In patient 2, however, stimulation of the right median nerve also evoked an equal contralateral response (fig 5). In patient 1, lower‐amplitude cortical responses were recorded contralaterally to the stimulus applied on either side. These were also recorded on left median nerve stimulation in patient 2, but also ipsilaterally to the stimulus, in an age‐matched control (fig 5).
Figure 5 Somatosensory evoked potentials of the median nerve. Recordings of the cortical responses (N20) were obtained simultaneously bilaterally. Left median nerve stimulation (Stim) evoked an N20 response ipsilateral in the patients with HGPPS (patients 1 and 2) and contralateral in the age‐matched control. Right median nerve stimulation evoked an equal bilateral response in patient 2, an ipsilateral response only in patient 1 and a normal contralateral response in the control.
Blink reflex
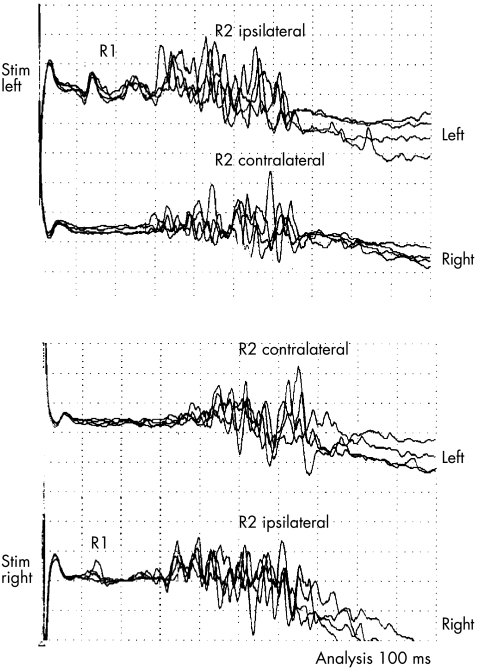
Stimulation on either side elicited a normal R1 ipsilateral to the stimulus and a normal R2 bilaterally (fig 6).
Figure 6 Blink reflex (patient 1). R1 was elicited ipsilaterally to the stimulus (Stim) and R2 on both sides.
Sympathetic skin response
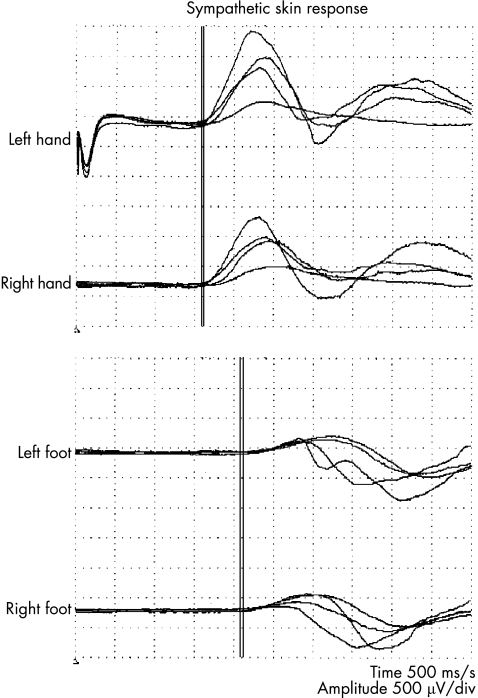
Normal responses were recorded on left median nerve stimulation from both palms and soles (fig 7).
Figure 7 Sympathetic skin response. Stimulation of the left median nerve (indicated by the stimulus artifact at the beginning of the first trace) evoked normal responses on both palms and soles (patient 1).
Audiograms
Conventional audiometry thresholds were in the normal range of values (0–15 dbHL) for the entire frequency range (125–8000 Hz) on the left side in both patients. However, on the right side, both brothers showed sensorineural hearing loss at high frequencies (>4 kHz).
Tympanometry
Measurement of the physical volume and the static compliance showed normal values bilaterally. The tympanogram showed normal pressures in the middle ear cavity bilaterally, with peak compliance between 0 and 50 daPa (tympanogram type A). The eustachian tube function tests (Valsalva–Toynbee, pressure‐swallow) showed normal function.
Acoustic reflex
Uncrossed and crossed acoustic reflexes could not be elicited bilaterally (on either side) even with the use of maximal stimulus intensities.
Brain stem auditory evoked potentials
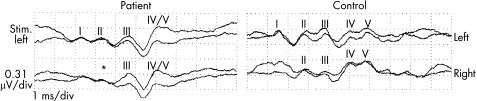
Waves I, II and III were found to be absent on right ear stimulation in both patients, indicating peripheral damage. On left ear stimulation, recordings from the left side showed waves I, II and III, and a complex of waves IV and V, whereas recordings from the right side showed an absent wave II. Waves III and a complex of waves IV and V were present (fig 8). The latency of wave I was prolonged.
Figure 8 Brain stem auditory evoked potentials. Simultaneous left and right recordings on left ear stimulation (Stim.). In patient 1 with HGPPS wave I was delayed and no obvious wave II (*) was recorded contralaterally.
Discussion
We carried out neuropsychological tests and neurophysiological investigations on two brothers homozygotic for the ROBO3 E319K mutation. Our MEP results, showing that stimulation of motor cortex evoked ipsilateral responses, are similar to previous observations,3,4 and suggest that the descending motor pathways to the upper and lower extremities were uncrossed in these patients. Also, EMG studies, obtained in patient 2, were consistent with uncrossed motor pathways to the neck muscles that are responsible for head rotation. These EMG recordings showed that rightward head rotation induced contraction in the left sternocleidomastoideus muscle without concomitant contraction in the right splenius capitis muscle (as would have been expected in the normal). However, in patient 1, a rightward head rotation induced contraction both of the left sternocleidomastoideus and of the right splenius capitis, thus suggesting crossover of the pyramidal tract to the neck muscles in this patient. These data indicate intrafamilial variability in the phenotypic expression of the same genetic defect (ROBO3 E319K mutation).
A similar intrafamilial variability was also apparent for the sensory system. Thus, although in patient 1 the obtained somatosensory evoked potentials were ipsilateral to the stimulus (suggesting uncrossed funiculus cuneatus as previously reported3,4), right‐sided stimuli in patient 2 evoked bilateral responses, suggesting a partial crossing of the right cuneatal tract. This possibility is supported by functional MRI results (unpublished data), which showed that, although movements of the left fingers induced activation of the left sensory cortex, movements of the right fingers induced activation of both the right and the left parietal cortices. A small N20 evoked ipsilateral to the stimulus in the control is believed to be volume conducted from the contralateral hemisphere as suggested by Kakigi et al,18 who studied 15 normal volunteers. Differentiation between the right and left cortical responses in tibial somatosensory evoked potential, when using a single midline recording electrode (Cz'), is not possible because of the vicinity of the left and right sensory foot areas in the midline of the scalp. Distinguishing could be possible when more recording channels are used.4
Our data differ from those of MacDonald et al4 and Jen et al,3 who found a complete lack of crossing of the dorsal column–medial lemniscal system. As the patients with HGPPS studied by those authors had different types of ROBO3 gene mutations (than the E319K substitution), the observed transfamilial variability probably relates to the distinct amino‐acid substitutions in the ROBO3 protein. However, our observations on the intrafamilial variability in the phenotypic expression of the same genetic defect (ROBO3 E319K mutation), as described earlier, suggest that additional factors are involved.
In contrast with the above data, the pathways for the blink reflex and for the SSRs seemed to be normally crossed in our patients. Indeed, the appearance of SSRs bilaterally on left median nerve stimulation indicates the presence of a crossed pathway involving the spinothalamic tract.19 Hosokawa et al20 and Terakawa et al21 have reported impaired light touch and pain sensation contralateral to the lesion in their patients with HGPPS who had had either a capsular or a putaminal haemorrhage, respectively, but their patients were not characterised at the genetic level. Regarding the blink reflex, stimulation on either side elicited a normal R1 ipsilaterally to the stimulus and an R2 bilaterally. The occurrence of the second response (R2), recorded contralaterally to the stimulus, indicates a midline crossing link between nucleus spinalis n. trigemini and the contralateral nucleus n. facialis.22
Evaluation of the auditory system using BAEPs showed that when the left ear was stimulated, waves I, II, III and a complex of waves IV and V were recorded ipsilaterally, whereas only waves III and a complex of waves IV and V were recorded contralaterally (fig 8). As the source of wave II is thought to be the nucleus cochlearis and that of wave III the nucleus of the superior olivary complex, failure to record wave II contralaterally indicates a lack of crossing of the axons that form the trapezoid body.23 Failure to record a crossed acoustic reflex, as shown here, is also consistent with an absent trapezoid body. On the other hand, the bilateral recording of waves III, IV and V on left ear stimulation indicates the presence of auditory pathways that span the brain stem rostrally of the trapezoid body (olivary complex, nucleus lemnisci lateralis, comissura colliculi inferiores). The presence, however, of peripheral damage of the auditory nerve (mainly on the right side) limits the interpretation of these findings.
Our patients with HGPPS showed no mirror movements, although these are known to occur in other disorders characterised by defective axonal crossing, such as Kallmann syndrome, Klippel–Feil syndrome and congenital hemiparesis.24,25 Transcranial magnetic stimulations in patients with Kallmann syndrome have evoked bilateral responses, with the ipsilateral response often being more pronounced, thus suggesting that the motor pathways are predominantly uncrossed.24 However, in contrast with HGPPS, in which both the motor and somatosensory pathways are uncrossed, the sensory pathways in Kallmann syndrome are normally crossed.24 This could relate to the fact that mirror movements occur in Kallmann syndrome but not in HGPPS.
The pathophysiology of the progressive scoliosis, a rather consistent feature of this disorder, remains unclear. Disturbances in the regulation of the muscle tone of paraspinal muscles, initiated by uncrossed pyramidal tract and perhaps rubrospinal tract, have been suggested.3,26
Despite their uncrossed or partially crossed motor, somatosensory and auditory pathways, our patients performed normally on manual dexterity, complex sensory and spatial–visuoperceptual functions, and on reading and general intelligence tests. Hence, crossing of motor and somatosensory pathways may not be essential for these complex functions in humans. The presence of a normal corpus callosum, a structure formed by the massive crossing of axons that connect the two cerebral hemispheres, may permit the integration of sensory input from the two hands and the cognitive processes that require interhemispheric connections,27 thus accounting for the above results.
Acknowledgements
We thank Dr Joanna Jen for her contribution to the analyses of the ROBO3 gene and Dr Giacomo P Comi for the mitochondrial DNA analysis.
Abbreviations
BAEP - brain stem auditory evoked potential
EMG - electromyography
FDI - first dorsal interosseus
HGPPS - horizontal gaze palsy and progressive scoliosis
SSR - sympathetic skin response
Footnotes
Competing interests: None declared.
References
- 1.Dretakis E K, Kondoyannis P N. Congenital scoliosis associated with encephalopathy in five children of two families. J Bone Joint Surg Am 1974561747–1750. [PubMed] [Google Scholar]
- 2.Jen J, Coulin C J, Bosley T M.et al Familial horizontal gaze palsy with progressive scoliosis maps to chromosome 11q23–25. Neurology 200259432–435. [DOI] [PubMed] [Google Scholar]
- 3.Jen J C, Chan W M, Bosley T M.et al Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science 20043041509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacDonald D B, Streletz L J, Al‐Zayed Z.et al Intraoperative neurophysiologic discovery of uncrossed sensory and motor pathways in a patient with horizontal gaze palsy and scoliosis. Clin Neurophysiol 2004115576–582. [DOI] [PubMed] [Google Scholar]
- 5.Bosley T M, Salih M A, Jen J C.et al Neurologic features of horizontal gaze palsy and progressive scoliosis with mutations in ROBO3. Neurology 2005641196–1203. [DOI] [PubMed] [Google Scholar]
- 6.Pieh C, Lengyel D, Neff A.et al Brainstem hypoplasia in familial horizontal gaze palsy and scoliosis. Neurology 200259462–463. [DOI] [PubMed] [Google Scholar]
- 7.Mori H, Fujishiro T, Hayashi N.et al Partially uncrossed pyramidal tracts shown by tractography in horizontal gaze palsy and scoliosis. Am J Roentgenol 2005184(Suppl)S4–S6. [DOI] [PubMed] [Google Scholar]
- 8.Ayres A J.Sensory integration and praxis test. Los Angeles: Western Psychological Services, 1989189–190.
- 9.Benton A L, Hamsher K S, Varney N R.et alContributions to neuropsychological assessment. New York: Oxford University Press, 1983310–312.
- 10.Gainotti G, Cianchetti C, Tiacci C. The influence of the hemispheric side of lesion on non verbal tasks of finger localization. Cortex 19728364–377. [DOI] [PubMed] [Google Scholar]
- 11.Reitan R M, Davidson L A.Clinical neuropsychology: current status and applications. New York: Winston, 1974230–231.
- 12.Lezak M D.Neuropsychological assessment. 3rd edn. New York: Oxford University Press, 1994341
- 13.Ruff R M, Parker S B. Gender‐ and age‐specific changes in motor speed and eye‐hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Motor Skills 1993761219–1230. [DOI] [PubMed] [Google Scholar]
- 14.Schenkenberg T, Bradford D C, Ajax E T. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 198030509–517. [DOI] [PubMed] [Google Scholar]
- 15.Dennis M, Edelstein K, Frederick J.et al Peripersonal spatial attention in children with spina bifida: associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia 2005432000–2010. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield R C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971997–113. [DOI] [PubMed] [Google Scholar]
- 17.Jewell G, McCourt M E. Pseudoneglect: a review and meta‐analysis of performance factors in line bisection tasks. Neuropsychologia 20003893–110. [DOI] [PubMed] [Google Scholar]
- 18.Kakigi R. Ipsilateral and contralateral SEP components following median nerve stimulation: effects of interfering stimuli applied to the contralateral hand. Electroencephalogr Clin Neurophysiol 198664246–259. [DOI] [PubMed] [Google Scholar]
- 19.Kimura J. Sympathetic skin response. Electrodiagnosis in diseases of nerve and muscle: principles and practice 3rd edn. Oxford: Oxford University Press, 2001115–117.
- 20.Hosokawa S, Tsuji S, Uozumi T.et al Ipsilateral hemiplegia caused by right internal capsule and thalamic hemorrhage: demonstration of predominant ipsilateral innervation of motor and sensory systems by MRI, MEP, and SEP. Neurology 1996461146–1149. [DOI] [PubMed] [Google Scholar]
- 21.Terakawa H, Abe K, Nakamura M.et al Ipsilateral hemiparesis after putaminal hemorrhage due to uncrossed pyramidal tract. Neurology 2000541801–1805. [DOI] [PubMed] [Google Scholar]
- 22.Kimura J.The blink reflex. Electrodiagnosis in diseases of nerve and muscle: principles and practice 3rd edn. Oxford: Oxford University Press, 2001409–411.
- 23.Chiappa K H. Brain stem auditory evoked potentials. In: Chiappa KH, ed. Evoked potentials in clinical medicine. 2nd edn. New York: Raven Press, 1990223–236.
- 24.Mayston M J, Harrison L M, Quinton R.et al Mirror movements in X‐linked Kallmann's syndrome. I. A neurophysiological study. Brain 19971201199–1216. [DOI] [PubMed] [Google Scholar]
- 25.Farmer S F. Mirror movements in neurology. J Neurol Neurosurg Psychiatry 2005761330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vulliemoz S, Raineteau O, Jabaudon D. Reaching beyond the midline: why are human brains cross wired? Lancet Neurol 2005487–99. [DOI] [PubMed] [Google Scholar]
- 27.Sack A T, Camprodon J A, Pascual‐Leone A.et al The dynamics of interhemispheric compensatory processes in mental imagery. Science 2005308702–704. [DOI] [PubMed] [Google Scholar]