Abstract
Background
Evoked potentials are used in the functional assessment of sensory and motor pathways. Their usefulness in monitoring the evolution of multiple sclerosis has not been fully clarified.
Objective
The aim of this longitudinal study was to examine the usefulness of multimodal evoked potential in predicting paraclinical outcomes of disease severity and as a prognostic marker in multiple sclerosis.
Methods
Eighty four patients with clinically definite multiple sclerosis underwent Expanded Disability Status Scale (EDSS) and functional system scoring at study entry and after a mean (standard deviation) follow‐up of 30.5 (11.7) months. Sensory and motor evoked potentials were obtained in all patients at study entry and at follow‐up in 64 of them, and quantified according to a conventional score.
Results
Cross‐sectionally, the severity of each evoked potential score significantly correlated with the corresponding functional system (0.32<R<0.60, p<0.01, for all but follow‐up visual evoked potential) and with EDSS (0.34<R<0.61; p<0.001 for all but brain stem evoked potential). EDSS significantly correlated with global evoked potential score severity (baseline R = 0.60, follow‐up R = 0.46, p<0.001). Using longitudinal analysis, only changes in somatosensory evoked potential scores were significantly correlated with changes of sensory functional system (R = 0.34, p = 0.006). However, patients with multiple sclerosis with disability progression at follow‐up had more severe baseline evoked potential scores than patients who remained stable. Patients with severe baseline global evoked potential score (higher than the median value) had a risk of 72.5% to progress on disability at follow‐up, whereas patients with multiple sclerosis with lower scores had a risk of only 36.3%.
Conclusions
These results suggest that evoked potential is a good marker of the severity of nervous damage in multiple sclerosis and may have a predictive value regarding the evolution of disability.
Evoked potentials have been used for more than 20 years in multiple sclerosis to objectify the involvement of sensory and motor pathways in patients with vague and indefinite disturbances, or to disclose clinically silent lesions.1 Although evoked potentials allow functional assessment of nervous conduction along clinically eloquent pathways, the pathophysiology of their abnormalities in multiple sclerosis is quite complex and not completely understood. The pathological substrates of functional deficits in multiple sclerosis are mainly represented by demyelination and axonal loss.2,3 Demyelination may determine slowing of conduction, failure to transmit impulses at high frequency, partial or complete conduction block and secondary axonal degeneration.4 The combination of all these phenomena may account for the pattern of evoked potential abnormalities found in individual patients. The value of evoked potentials in the diagnosis of multiple sclerosis has been greatly reduced after the advent of magnetic resonance imaging (MRI), because of the higher sensitivity of MRI to subclinical lesions.1 As a matter of fact, although the McDonald criteria for multiple sclerosis diagnosis,5 largely based on MRI findings, have been criticised,6 only visual evoked potentials are viewed as contributing to the diagnosis of multiple sclerosis.5
Although there is a general agreement that evoked potentials correlate with function in somatosensory,7,8 visual9 or motor pathways10 and the combination of evoked potential abnormalities correlates with disability,11,12 the usefulness of multimodal evoked potentials in monitoring the evolution of multiple sclerosis has not been yet clarified. Conflicting results have been reported on the correlation between clinical and evoked potential changes, which were absent or mild in some studies13,14,15,16,17 carried out on small samples and with short follow‐up. A better relationship has been found in other studies,11,18,19,20,21 mainly because of a higher number of patients studied and longer follow‐up duration. Moreover, the use of conventional scores to quantify the severity of neurophysiological abnormalities allows us to combine the results of multimodal evoked potential in a global score, reflecting the global impairment of function.12 The responsiveness of these scales during the various phases of the disease has yet to be defined. The aim of this study was to evaluate whether neurophysiological abnormalities may parallel the clinical disease evolution in a cohort of patients with established multiple sclerosis, by means of multimodal evoked potentials quantified using a conventional score.
Methods
Data were collected from patients who referred to the multiple sclerosis centre of our department (Department of Neurology, Clinical Neurophysiology and Neurorehabilitation, University Vita‐Salute, Milan, Italy) for routine clinical and neurophysiological evaluations. Inclusion criteria were (1) diagnosis of clinically definite multiple sclerosis22; (2) complete neurological exam with disability rating using the Expanded Disability Status Scale (EDSS) and Kurtzke's functional system scores23; (3) multimodal evoked potentials (visual, brain stem auditory, somatosensory and motor to the four limbs) separated by an interval of <3 weeks from clinical examination; (4) second clinical examination after 2.5–5 years. Patients with a relapse within 2 months before baseline clinical evaluation were not included. Using these criteria, we selected 84 patients with multiple sclerosis (43 with relapsing remitting, 28 secondary progressive and 13 primary progressive disease course24) with clinical follow‐up after a mean (standard deviation (SD)) of 30 (11.7) months. For 64 (76%) patients, a second complete multimodal evoked potential battery within 3 weeks from the second clinical examination was also available.
Multimodal evoked potentials
Multimodal visual, auditory, somatosensory and motor evoked potentials to the four limbs were obtained according to previously published guidelines.25 Somatosensory evoked potentials (SEPs) were obtained on electrical stimulation of the median nerve at the wrist for the upper limb and at the tibial nerve for the lower limb. One patient had median nerve SEP for one limb only. Latencies of the main peripheral, spinal and cortical components were measured, and central conduction time was calculated as the difference between cortical and spinal latencies (for tibial nerve SEP, latencies were corrected by height).
Visual evoked potentials (VEPs) to reversal achromatic checks (each subtending 30 and 15 min of visual angle (min arc)) were recorded over Oz of the 10–20 international EEG system, with Cz as the reference. Latency and amplitude of the P100 component were measured.
Brain stem auditory evoked potentials (BAEPs) to clicks at 85 dB normal hearing level were recorded at the Cz electrode referred to the ipsilateral and contralateral ear. The latency of the main peaks I, III and V and the I:V amplitude ratio were measured.
Motor evoked potentials (MEPs) to the four limbs were obtained using a Cadwell MS10 magnetic stimulator with a round coil (outer diameter 12 cm). The coil was placed tangentially to the scalp, with its centre over the vertex. Patients were asked to contract slightly the target muscles (abductor pollicis brevis and abductor hallucis) at about 20% of the maximum voluntary effort to facilitate motor responses. Spinal roots were stimulated at C6–C7 and L4–L5 spaces, while recording from the same muscles. Central motor conduction time was measured as the difference between total and peripheral motor conduction time.
Evoked potential analysis
For all evoked potential modalities, latencies (and, where measured, amplitudes) were compared with normative data obtained in our laboratory. Absolute or interside difference values exceeding 2.5 SD from normal values or absence or morphological abnormality of a major component were considered abnormal. Evoked potential abnormalities were quantified according to a conventional 4‐point graded ordinal score (0 = normal; 1 = increased latency; 2 = increased latency plus morphological abnormalities of a major component; 3 = absence of a major component) modified from Fukutake et al.8 For each modality, the evoked potential score was the sum of the scores in the two sides (in the patient with median SEP on one side only, the related score was multiplied by two). We calculated the global evoked potential score as the sum of the left and right BAEPs (from 0 to 6), VEPs (from 0 to 6), and of the left and right upper and lower SEPs (from 0 to 12) and MEPs (from 0 to 12) scores. This score ranges from 0 to 36, with higher values representing a more severe evoked potential involvement. Evoked potentials were independently evaluated by two neurophysiologists (LL and SM) blinded to the clinical status of the patients and to the timing of the evaluation; evoked potentials with discordant scoring of morphology (<10%) were reconsidered by the two examiners to reach a consensus.
Statistical analysis
Statistical analysis was carried out by means of non‐parametric tests, as ordinal scales were used (EDSS and evoked potential score). Ordinal variables were tested with the Kruskal–Wallis and Mann–Whitney U tests or the Wilcoxon matched pair test; categorical variables were tested with Fisher's exact test or McNemar's paired test. Correlations were tested using Spearman's rank correlation coefficient. A p value of 0.05 was used as cut‐off value. SPSS V.10.1 was used.
Results
Table 1 reports the clinical findings in the whole cohort and in the three clinical subgroups. As expected, EDSS did not significantly differ between the primary progressive and secondary progressive groups, whereas it was lower in patients with relapsing remitting disease compared with the whole group of patients with progressive disease.
Table 1 Median values (standard deviations) of clinical disability (Kurtzke's EDSS and functional systems) in the whole multiple sclerosis group and in the different disease courses: primary progressive; relapsing remitting; secondary progressive; progressive.
| MS (n = 84) | PP (n = 13) | RR (n = 43) | SP (n = 28) | SP v PP | Progressive v RR | |
|---|---|---|---|---|---|---|
| Age (years) | 37.8 (9.9) | 43.8 (6.9) | 33.7 (9.4) | 41.0 (9.5) | NS | <0.001 |
| F/M | 51/33 | 5/8 | 28/15 | 18/10 | NS | NS |
| MS duration (months) | 90.1 (77.2) | 54.5 (65.4) | 85.1 (71.3) | 112.7 (85.4) | 0.04 | NS |
| EDSS | 3.5 (1–8) | 5.5 (2–6.5) | 3 (1–6) | 5 (2.5–8) | NS | <0.001 |
| Pyramidal FS | 3 (0–5) | 3 (1–4) | 2 (0–5) | 3 (1–5) | NS | <0.001 |
| Brain stem FS | 1 (0–3) | 2 (0–3) | 1 (0–3) | 2 (0–3) | NS | NS |
| Sensory FS | 1 (0–3) | 2 (0–3) | 1 (0–3) | 2 (0–3) | NS | 0.03 |
| Visual FS | 0 (0–6) | 0 (0–3) | 0 (0–6) | 1 (0–6) | NS | 0.03 |
EDSS, Expanded Disability Status Scale; F, female; FS, functional system; M, male; MS, multiple sclerosis; NS, not significant; PP, primary progressive; progressive, pooled secondary progressive and primary progressive; RR, relapsing remitting; SP, secondary progressive.
p Values of differences (right column) represent contrasts by Mann–Whitney U test.
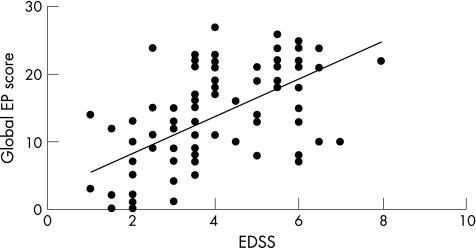
The percentage frequencies of evoked potential abnormalities (table 2) and the conventional evoked potential scores (table 3) at baseline did not significantly differ between the primary progressive and secondary progressive groups, whereas they were significantly higher in the progressive compared with the relapsing remitting group (Mann–Whitney; p⩽0.03) except for upper limb SEP and BAEP. A significant correlation (Spearman's rank correlation coefficient; R = 0.60, p<0.001) was present between the global evoked potential score and EDSS (fig 1).
Table 2 Percentage frequency of abnormalities of evoked potentials at basal evaluation in the whole multiple sclerosis group and in the different disease courses: primary progressive; relapsing remitting; secondary progressive; progressive.
| n = 84 | MS (%) | PP (%) | RR (%) | SP (%) | Progressive v RR |
|---|---|---|---|---|---|
| SEP‐UL | 53.6 | 69.2 | 44.2 | 60.7 | NS |
| SEP‐LL | 82.1 | 100 | 69.8 | 92.9 | 0.002 |
| SEP | 85.7 | 100 | 76.7 | 92.9 | 0.02 |
| MEP‐UL | 72.6 | 84.6 | 55.8 | 92.9 | <0.001 |
| MEP‐LL | 77.4 | 92.3 | 60.5 | 96.4 | <0.001 |
| MEP | 78.6 | 92.3 | 62.8 | 96.4 | <0.001 |
| BAEP | 64.0 | 53.8 | 41.9 | 42.9 | NS |
| VEP | 77.4 | 92.3 | 67.4 | 85.7 | 0.03 |
| Any EP | 94.0 | 100 | 88.4 | 100 | 0.02 |
EP, evoked potential; LL, lower limb; MEP, motor evoked potential; MS, multiple sclerosis; NS, not significant; PP, primary progressive; progressive, pooled secondary progressive and primary progressive; RR, relapsing remitting; SEP, somatosensory evoked potential; SP, secondary progressive; UL, upper limb; VEP, visual evoked potential.
p Values of differences (right column) represent Fisher's exact test.
Table 3 Basal evoked potential scores in the whole multiple sclerosis group and in the different disease courses: primary progressive; relapsing remitting; secondary progressive; progressive.
| n = 84 | MS | PP | RR | SP | Progressive v RR |
|---|---|---|---|---|---|
| SEP‐UL | 1.8 (2.1) | 2.1 (1.9) | 1.7 (2.4) | 1.9 (1.9) | NS |
| SEP‐LL | 3.4 (2.3) | 5.2 (1.4) | 2.5 (2.3) | 4.1 (2.1) | <0.001 |
| SEP | 5.3 (3.7) | 7.2 (2.4) | 4.2 (3.9) | 6.0 (3.3) | 0.004 |
| MEP‐UL | 1.7 (1.4) | 2.0 (1.4) | 1.2 (1.3) | 2.4 (1.2) | <0.001 |
| MEP‐LL | 2.3 (1.8) | 3.3 (1.9) | 1.5 (1.6) | 3.1 (1.4) | <0.001 |
| MEP | 4.1 (3.0) | 5.3 (3.2) | 2.7 (2.8) | 5.5 (2.4) | <0.001 |
| VEP | 2.8 (2.1) | 3.2 (1.5) | 2.3 (2.2) | 3.5 (1.9) | 0.01 |
| BAEP | 1.2 (1.7) | 1.5 (1.9) | 1.2 (1.8) | 1.1 (1.5) | NS |
| Global | 13.4 (7.5) | 17.2 (5.5) | 10.4 (7.8) | 16.5 (6.0) | <0.001 |
BAEP, brain stem auditory evoked potential; LL, lower limb; MEP, motor evoked potential; MS, multiple sclerosis; NS, not significant; PP, primary progressive; progressive, pooled secondary progressive and primary progressive; RR, relapsing remitting; SEP, somatosensory evoked potential; SP, secondary progressive; UL, upper limb; VEP, visual evoked potential.
p Values of differences (right column) represent contrasts by the Mann–Whitney test.
Figure 1 Basal global evoked potential (EP) score versus basal Expanded Disability Status Scale in the whole cohort of patients with multiple sclerosis (n = 84). R = 0.60; p<0.001 (Spearman correlation coefficient).
Data on follow‐up were available for 64 patients. The 20 patients with multiple sclerosis lost at follow‐up (8 primary progressive, 3 relapsing remitting and 9 secondary progressive) were significantly older than the remaining patients (mean age (SD) 43.7 (8.6) and 36 (9.7) years, respectively; p = 0.003, Student's t test). The correlation between global evoked potential score and EDSS and between the evoked potential score for each modality (except BAEP) with EDSS, and their related functional system was present also at the final evaluation in the 64 patients who completed the follow‐up (table 4).
Table 4 Correlation between basal, follow‐up and longitudinal delta values (difference between final and basal) of evoked potential scores and their related functional system (visual, somatosensory, pyramidal) and of evoked potential scores and Expanded Disability Status Scale in the whole cohort of patients with multiple sclerosis who underwent both basal and follow‐up evaluations.
| n = 64 | EDSS basal | EDSS final | Delta (final−basal) | FS basal | FS final | Delta (final−basal) |
|---|---|---|---|---|---|---|
| SEP | R = 0.55 (<0.001) | R = 0.41 (0.001) | R = 0.15 (NS) | R = 0.46 (<0.001) | R = 0.50 (<0.001) | R = 0.34 (0.006) |
| MEP | R = 0.50 (0.001) | R = 0.61 (<0.001) | R = 0.13 (NS) | R = 0.55 (<0.001) | R = 0.60 (<0.001) | R = 0.05 (NS) |
| VEP | R = 0.46 (<0.001) | R = 0.34 (0.006) | R = −0.10 (NS) | R = 0.47 (<0.001) | R = 0.18 (NS) | R = 0.01 (NS) |
| BAEP | R = 0.10 (NS) | R = 0.06 (NS) | R = 0.09 (NS) | R = 0.32 (0.01) | R = 0.33 (0.007) | R = 0.12 (NS) |
| Global EP | R = 0.60 (<0.001) | R = 0.46 (<0.001) | R = 0.18 (NS) |
BAEP, brain stem auditory evoked potential; EDSS, Expanded Disability Status Scale; EP, evoked potential; FS, functional system; LL, lower limb; MEP, motor evoked potential; NS, not significant; SEP, somatosensory evoked potential; UL, upper limb; VEP, visual evoked potential.
R and p values refer to Spearman's correlation coefficient.
On carrying out the latter analysis on the relapsing remitting and progressive groups separately at baseline and follow‐up in the relapsing remitting subgroup the correlation between BAEP and the related functional system was not significant both at baseline and follow‐up. In the progressive subgroup, all the correlations between evoked potential and EDSS scores were not significant, with the exception of that between MEP and EDSS score at final evaluation (R = 0.53, p = 0.008), whereas the correlation between MEP or SEP and the related functional system was significant at final and basal evaluations (R = 0.53 and 0.48, p = 0.007 and 0.002, respectively).
As regards longitudinal analysis, the frequency of abnormalities (table 5) increased from baseline to final evaluations for all the evoked potential modalities, but was significant only for upper limb SEP (McNemar test; p = 0.03), whereas the conventional evoked potential scores (table 6) significantly worsened for all modalities (Wilcoxon matched pairs test). At separate subgroup analysis the longitudinal correlation between changes in SEP and the related functional system was significant in the progressive group only (R = 0.43, p = 0.04).
Table 5 Percentage abnormalities of evoked potential in the whole cohort of patients with multiple sclerosis who underwent both basal and follow‐up evaluations.
| n = 64 | Basal (%) | Final (%) | p Value |
|---|---|---|---|
| SEP‐UL | 51.6 | 65.6 | 0.03 |
| SEP‐LL | 81.3 | 85.9 | NS |
| SEP | 84.4 | 89.1 | NS |
| MEP‐UL | 68.8 | 70.3 | NS |
| MEP‐LL | 71.9 | 79.7 | NS |
| MEP | 73.4 | 79.7 | NS |
| VEP | 75.0 | 82.8 | NS |
| BAEP | 40.6 | 50.0 | NS |
| Global EP | 92.2 | 96.9 | NS |
BAEP, brain stem auditory evoked potential; EP, evoked potential; LL, lower limb; MEP, motor evoked potential; NS, not significant; SEP, somatosensory evoked potential; UL, upper limb; VEP, visual evoked potential.
p Values of differences (right column) represent McNemar's test.
Table 6 Evoked potential baseline and final scores in the whole cohort of patients with multiple sclerosis who performed both basal and follow‐up evaluations.
| n = 64 | Baseline | Final | p Value |
|---|---|---|---|
| SEP‐UL | 1.8 (2.2) | 2.5 (2.3) | 0.007 |
| SEP‐LL | 3.7 (2.3) | 3.8 (2.3) | 0.04 |
| SEP | 5.0 (3.8) | 6.3 (3.9) | 0.004 |
| MEP‐UL | 1.6 (1.4) | 2.0 (1.7) | 0.003 |
| MEP‐LL | 2.1 (1.8) | 2.9 (2.0) | <0.001 |
| MEP | 3.7 (3.0) | 4.9 (3.5) | <0.001 |
| VEP | 2.7 (2.1) | 3.1 (2.1) | 0.02 |
| BAEP | 1.2 (1.8) | 1.6 (1.9) | 0.02 |
| Global EP | 12.6 (7.6) | 15.9 (8.8) | <0.001 |
BAEP, brain stem auditory evoked potential; EP, evoked potential; LL, lower limb; MEP, motor evoked potential; SEP, somatosensory evoked potential for the lower limb; SEP, somatosensory evoked potential for the upper limb; UL, upper limb; VEP, visual evoked potential.
p Values of differences (right column) represent Mann–Whitney test.
We also explored the relationship between the severity of evoked potential involvement at basal evaluation and the risk of disability progression at follow‐up, defined as an increase of at least 1 EDSS point if baseline EDSS was ⩽5.5, and of at least 0.5 point if baseline EDSS was >5.5.26 Patients with a more severe evoked potential involvement at baseline, represented by a global evoked potential score ⩾13, which was its median value, had a risk of 72.5% for progression to disability at the follow‐up examination, whereas patients with multiple sclerosis with less severe evoked potential involvement at baseline, corresponding to a global evoked potential score ⩽14, had a much lower risk of 36.3% (table 7), corresponding to an odds ratio (OR) of 4.6 95% confidence interval (CI 1.8 to 11.6). This leads to a positive predictive value of 72.5% and a negative predictive value of 63.6% of the baseline global evoked potential score, categorised according to its median value.
Table 7 Predictive value of evoked potential global score at basal examination on Expanded Disability Status Scale (EDSS) progression at follow‐up, defined as an increase of at least 1 (if baseline EDSS <5.5) and of at least 0.5 (if baseline EDSS ⩾5.5).
| EP >13 | EP ⩽13 | Total | PV | |
|---|---|---|---|---|
| EDSS+ | 29 | 16 | 45 | 0.725 |
| EDSS− | 11 | 28 | 39 | 0.636 |
| Total | 40 | 44 | 84 |
EDSS, Expanded Disability Status Scale; EP, evoked potential; PV, predictive value.
The EP global score was categorised into two groups according to the median value: ⩽13 (less involved EP) and >13 (more involved EP). χ2 analysis: p = 0.001; odds ratio = 4.6 (1.8–11.6).
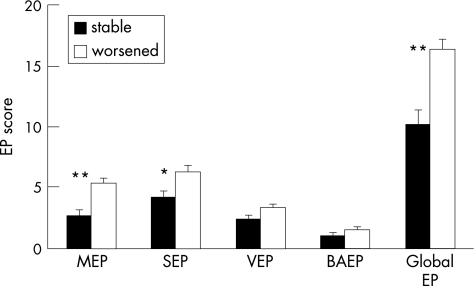
These data were confirmed by the fact that patients with multiple sclerosis whose condition worsened at follow‐up had significantly worse somatosensory (Mann–Whitney test; p = 0.01), motor (p<0.001) and global (p<0.001) evoked potential scores at baseline compared with patients without clinical worsening (fig 2).
Figure 2 Basal evoked potential (EP) scores in patients with stable (black bars) and worsened (white bars) disability at follow‐up. Expanded Disability Status Scale (EDSS) was considered as worsened if increased by 1 point (if basal EDSS <5.5) or increased by 0.5 points (if baseline EDSS ⩾5.5). *p = 0.01; **p<0.001 (Mann–Whitney test). BAEP, brain stem auditory evoked potential; MEP, motor evoked potential; SEP, somatosensory evoked potential; VEP, visual evoked potential.
Discussion
Cross‐sectional analysis
Consistent with previous literature,27,28,29,30 VEP, lower limb SEP and MEP were the most frequently involved evoked potentials in multiple sclerosis. These findings may be explained by a higher susceptibility of optic nerve fibres to multiple sclerosis lesions, confirmed by pathological studies,31 and by a higher probability of involvement of longer pathways such as sensorimotor projections to the lower limbs. We found more frequent and severe evoked potential abnormalities in progressive forms of the disease compared with the relapsing remitting form; this finding cannot be explained by the older age of patients with progressive multiple sclerosis included in our study, because central conduction time, different from peripheral conduction time, is only mildly affected by age.32 Moreover, according to previous studies,8,9,12,33,34,35,36,37 we found a marked correlation between the severity of abnormality of each evoked potential modality (except auditory), reflected by the evoked potential score, and EDSS or the corresponding functional system. These results indicate that evoked potentials in patients with multiple sclerosis correlate well with disability and with the severity of involvement of sensory and motor pathways.
Longitudinal analysis
Conflicting results have been reported on the correlation between clinical and evoked potential changes, being absent or equivocal in some studies,13,14,15,16,17 whereas a good correlation has been found in others.11,18,19,20,21
Such different results can be determined by the use of different measures for quantifying the involvement of evoked potentials, as well as by the type of enrolled patients (whether relapsing remitting or progressive) and by the duration of follow‐up.
In our study, the analysis of clinical and instrumental follow‐up data has shown a partial correlation between clinical worsening and evoked potential score changes. When assessed in terms of difference between the final and baseline evoked potential and EDSS scores, longitudinal correlation was significant only for SEP, different from previous reports.11,12,16 In 11 patients with chronic progressive multiple sclerosis followed up over 1.5 years, significant changes in VEP latencies but not in BAEP or MRI T2 lesion load were found.16 In a cohort of patients with relapsing remitting disease followed up for 2 years, a significant correlation between a global measure of evoked potential abnormalities and EDSS, both for cross‐sectional and longitudinal evaluations, has been reported.12 Fuhr et al11 found cross‐sectional and longitudinal correlations between combined VEP and MEP latency z‐score and EDSS in a group of 30 patients with multiple sclerosis (most of whom were relapsing remitting), who had been followed up for 2 years.
The reason why SEPs are most closely related to EDSS change in our study could be that spinal cord involvement is overweighted in EDSS, particularly at scores over 3.5,24 which represented almost half of our patients. In contrast, the lack of longitudinal correlation of the other evoked potentials with clinical disability could be determined by a ceiling effect of evoked potential abnormalities. This is underlined by the fact that in our study, cross‐sectional correlations were higher at baseline than at follow‐up evaluation, and also by the high proportion of patients with progressive disease, with higher evoked potential scores, of which the highest refers to absence of a major evoked potential component, not susceptible to further worsening. This is also determined by the fact that, in analogy to other studies,8,9,37 we used conventional scores taking into account the absence of a major component, thus avoiding excluding from the analysis patients with severe evoked potential abnormalities, which are more likely to present a severe disability. It is, however, worth remembering that the limited sample size of the progressive patient subgroup might be a limiting factor when interpreting the results of the separate correlation analysis carried out in these patients.
However, and consistent with previous reports,11 the global evoked potential score in our study showed a predictive value with regard to future clinical evolution, indicated by a higher risk of disability worsening in patients with higher evoked potentials score, and by more severe basal evoked potential scores in patients with clinical worsening at follow‐up compared with stable or improved patients.
It is worthwhile mentioning that our global evoked potential score is weighted towards sensory evoked potentials and MEPs, which count double that of visual and auditory. Moreover, a marked discrepancy between evoked potentials in worsened and stable patients was present for somatosensory and motor modalities, confirming the importance of spinal cord involvement in the development of disability. Our finding that detection of involvement of eloquent sensory or motor pathways is predictive for the development of future disability in patients with multiple sclerosis is consistent with MRI findings showing continuous optic nerve atrophy after optic neuritis, not evident in the unaffected eye.38 The interpretation of these findings is not unequivocal, because the relationship between inflammation, demyelination and axonal damage in multiple sclerosis still needs to be clarified. Recent pieces of evidence39,40 indicate that the use of measures derived from quantitative MRI‐based techniques provides useful predictive information for the medium‐term clinical disease evolution, most probably because these techniques can quantify the severity of structural and irreversible multiple sclerosis‐related tissue damage. Studies incorporating both multimodal evoked potential and quantitative MRI evaluations are, therefore, warranted to clarify whether a multiparametric approach may improve our prognostic ability in the investigation of patients with established multiple sclerosis.
In conclusion, by showing a good correlation between clinical assessment and evoked potential, our results support the usefulness of neurophysiological data both in monitoring the natural history of the disease and in predicting the development of future disability. The integration of MRI and evoked potential data may give us new prospects on the knowledge of pathological processes underlying multiple sclerosis evolution and, if validated, could prove useful in monitoring the effects of symptomatic and disease‐modifying treatments.
Acknowledgements
This study was supported by a grant from the Instituto Superiore di Sanità (National Ministry of Health, Rome, Italy. Protocol number 96/J/T44)
Abbreviations
BAEP - brain stem auditory evoked potential
EDSS - Expanded Disability Status Scale
MEP - motor evoked potential
MRI - magnetic resonance imaging
SEP - somatosensory evoked potential
VEP - visual evoked potential
Footnotes
Competing interests: None declared.
References
- 1.Comi G, Locatelli T, Leocani L.et al Can evoked potentials be useful in monitoring multiple sclerosis evolution? Electroencephalogr Clin Neurophysiol Suppl 199950349–357. [PubMed] [Google Scholar]
- 2.Trapp B D, Peterson J, Ransohoff R M.et al Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998338278–285. [DOI] [PubMed] [Google Scholar]
- 3.Bitsch A, Schuchardt J, Bunkowski S.et al Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 20001231174–1183. [DOI] [PubMed] [Google Scholar]
- 4.McDonald W I, Sears T A. The effects of experimental demyelination on conduction in the central nervous system. Brain 197093583–598. [DOI] [PubMed] [Google Scholar]
- 5.McDonald W, Compston D, Edan G.et al Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 200150121–127. [DOI] [PubMed] [Google Scholar]
- 6.Poser C M. The diagnosis and management of multiple sclerosis. Acta Neurol Scand 2005112199–201. [DOI] [PubMed] [Google Scholar]
- 7.Leocani L, Martinelli V, Natali‐Sora M G.et al Somatosensory evoked potentials and sensory involvement in multiple sclerosis: comparison with clinical findings and quantitative sensory tests. Mult Scler 20039275–279. [DOI] [PubMed] [Google Scholar]
- 8.Fukutake T, Kuwabara S, Kaneko M.et al Sensory impairments in spinal multiple sclerosis: a combined clinical, magnetic resonance imaging and somatosensory evoked potential study. Clin Neurol Neurosurg 1998100199–204. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock‐Guttman B, Baier M, Stockton R.et al Pattern reversal visual evoked potentials as a measure of visual pathway pathology in multiple sclerosis. Mult Scler 20039529–534. [DOI] [PubMed] [Google Scholar]
- 10.Van der Kamp W, Maertens de Noordhout A, Thompson P D.et al Correlation of phasic muscle strength and corticomotoneuron conduction time in multiple sclerosis. Ann Neurol 1991296–12. [DOI] [PubMed] [Google Scholar]
- 11.Fuhr P, Borggrefe‐Chappuis A, Schindler C.et al Visual and motor evoked potentials in the course of multiple sclerosis. Brain 20011242162–2168. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor P, Marchetti P, Lee L.et al Evoked potential abnormality scores are a useful measure of disease burden in relapsing‐remitting multiple sclerosis. Ann Neurol 199844404–407. [DOI] [PubMed] [Google Scholar]
- 13.Matthews V B, Small D G. Serial recording of visual and somatosensory evoked potentials in multiple sclerosis. J Neurol Sci 19794011–21. [DOI] [PubMed] [Google Scholar]
- 14.Aminoff M J, Davis S L, Panitch H S. Serial evoked potentials studies in patients with definite multiple sclerosis. Arch Neurol 1984411197–1202. [DOI] [PubMed] [Google Scholar]
- 15.Davis S L, Aminoff M J, Panitch H S. Clinical correlations of serial somatosensory evoked potentials in multiple sclerosis. Neurology 198535359–365. [DOI] [PubMed] [Google Scholar]
- 16.Sater R A, Rostami A M, Galetta S.et al Serial evoked potential studies and MRI imaging in chronic progressive multiple sclerosis. J Neurol Sci 199917179–83. [DOI] [PubMed] [Google Scholar]
- 17.De Weerd A W, Jonkman E J. Changes in visual and short latency somatosensory evoked potentials in patients with multiple sclerosis. In: Courjon J, Mauguière F, Revol N, eds. Clinical applications of evoked potentials in neurology. New York: Raven Press, 1982527–534. [PubMed]
- 18.Walsh J C, Garrick R, Cameron J.et al Evoked potentials changes in clinically definite multiple sclerosis: a two years follow up study. J Neurol Neurosurg Psychiatry 198245494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghezzi A, Zaffaroni M, Caputo D.et al Evaluation of evoked potentials and lymphocyte subsets as posible markers of multiple sclerosis: one year follow‐up of 30 patients. J Neurol Neurosurg Psychiatry 198649913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuwer M R, Packwood J W, Myers L W.et al Evoked potentials predict the clinical changes in a multiple sclerosis drug study. Neurology 1987371754–1761. [DOI] [PubMed] [Google Scholar]
- 21.Andersson T, Siden A. Multimodality evoked potentials and neurological phenomenology in patients with multiple sclerosis and potentially related conditions. Electromyogr Clin Neurophysiol 199131109–117. [PubMed] [Google Scholar]
- 22.Poser C M, Paty D W, Scheinberg L.et al New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 198313227–231. [DOI] [PubMed] [Google Scholar]
- 23.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 24.Lublin F D, Reingold S C. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 199646907–911. [DOI] [PubMed] [Google Scholar]
- 25.Deuschl G, Eisen A. Recommendations for the practice of clinical neurophysiology: Guidelines of the International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol 199952(Suppl)192–211. [PubMed] [Google Scholar]
- 26. European study group on interferon beta‐1b in secondary progressive MS. Placebo‐controlled multicenter randomised trial of interferon beta‐1b in treatment of secondary progressive multiple sclerosis. Lancet 19983521491–1497. [PubMed] [Google Scholar]
- 27.Chiappa K H. Pattern shift visual, brainstem auditory and short latency somatosensory evoked potentials in multiple sclerosis. Neurology 198030110–123. [DOI] [PubMed] [Google Scholar]
- 28.Khosbin S, Hallett M. Multimodality evoked potentials and blink reflex in multiple sclerosis. Neurology 198131138–144. [DOI] [PubMed] [Google Scholar]
- 29.Trojaborg W, Petersen E. Visual and somatosensory evoked potentials in multiple sclerosis. J Neurol Neurosurg Psychiatry 197942323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comi G, Martinelli V, Medaglini S.et al Correlation between multimodal evoked potentials and magnetic resonance imaging in multiple sclerosis. J Neurol 19892364–8. [DOI] [PubMed] [Google Scholar]
- 31.Dawson J W. The histology of disseminated sclerosis (part 1, 2, 3). Edinburgh Med J N S 191617311–410. [Google Scholar]
- 32.Onofrj M, Thomas A, Iacono D.et al Age‐related changes of evoked potentials. Neurophysiol Clin 20013183–103. [DOI] [PubMed] [Google Scholar]
- 33.Celesia G G. Visual evoked potentials in clinical neurology. In: Aminoff M, ed. Electrodiagnosis in clinical neurology. New York: Churchill‐Livingstone, 1992467–489.
- 34.Ingram D A, Thomson A J, Swash M. Central motor conduction in multiple sclerosis: evaluation of abnormalities revealed by transcutaneous magnetic stimulation of the brain. J Neurol Neurosurg Psychiatry 198851487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comi G, Locatelli T, Leocani L. Confronto tra potenziali evocati somatosensoriali e test quantitativi delle sensibilità nei pazienti con sclerosi multipla. In: Comi G, ed. I potenziali evocati nella sclerosi multipla. Italia: Springer‐Verlag, 199585–90.
- 36.Filippi M, Campi A, Mammi S.et al Brain magnetic resonance imaging and multimodal evoked potentials in benign and secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 19955831–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koehler J, Faldum A, Hopf H C. EDSS correlated analysis of median nerve somatosensory evoked potentials in multiple sclerosis. Neurol Sci 200021217–221. [DOI] [PubMed] [Google Scholar]
- 38.Hickman S J, Brierley C M, Brex P A.et al Continuing optic nerve atrophy following optic neuritis: a serial MRI study. Mult Scler 20028339–342. [DOI] [PubMed] [Google Scholar]
- 39.Fisher E, Rudick R A, Simon J H.et al Eight‐year follow‐up study of brain atrophy in patients with MS. Neurology 2002591412–1420. [DOI] [PubMed] [Google Scholar]
- 40.Rovaris M, Agosta F, Sormani M P.et al Conventional and magnetization transfer MRI predictors of clinical multiple sclerosis evolution: a medium‐term follow‐up study. Brain 20031262323–2332. [DOI] [PubMed] [Google Scholar]