Abstract
Spina bifida is a multifaceted neurological condition with complex neuropsychological sequelae. The cognitive outcome in spina bifida has frequently been attributed to the severity of the hydrocephalus. However, because of complex neuropathology, the influence of hydrocephalus alone does not sufficiently explain the deficits in the cognitive profile in spina bifida. To date, little is known of the role of Arnold‐Chiari‐II malformation (ACM) in the cognitive profile of these patients. Aim of the current study is to delineate the specific contribution of the ACM in spina bifida by comparing children with ACM and those without ACM. 46 children between 6 and 15 years of age underwent a neuropsychological assessment covering intelligence and a wide range of cognitive functions, such as visuo‐motor processing, attention, memory, word fluency and speed of information processing. Comparisons were made between patients with ACM (ACM+) and those without ACM (ACM−); all children with ACM+ also had hydrocephalus. Confounding effects of global cognitive impairment were excluded, such that groups were matched on verbal IQ. Because of complex neuropathology, which is inherent to spina bifida, the method applied was based on a comparison of cognitive profiles of the study group with profiles of patients with cerebellar damage and hydrocephalus found in the literature. Impaired visual analysis and synthesis, verbal memory, and verbal fluency, even after correction for global cognitive impairment, were observed in children with ACM. The hypothesis that in addition to impairment in visual analysis and synthesis, which are related to both hydrocephalus and ACM, specific deficiencies in verbal memory and fluency may be attributed to ACM is supported.
Spina bifida is a heterogeneous congenital disorder with complex physical and neuropsychological symptomatology. It is associated with developmental anomalies of both the spine and the central nervous system. Different forms of spina bifida can be distinguished, varying from mild to severe. The most common form is myelomeningocele. Nearly all children with myelomeningocoele develop a hydrocephalus and have Arnold‐Chiari‐II malformation (ACM). ACM includes a low tentorium insertion, herniation of the posterior fossa content and beaking of the mesencephalic tectum.1
Owing to improved diagnosis and treatment, children with spina bifida and hydrocephalus (SBH) are more likely to have a total IQ (TIQ) within the normal range than in previous decades.2,3 Until now, the cognitive impairments in spina bifida have often been attributed to the severity of the hydrocephalus and associated complications such as shunt revisions, infections or seizures.4,5,6,7,8,9,10,11 In spina bifida, however, hydrocephalus and ACM do not occur independently. Consequently, the influence of hydrocephalus alone cannot sufficiently explain the deficits in the cognitive profile of these patients. Until now, little research has been conducted on the specific role of ACM. Some evidence suggests that ACM influences motor and cognitive development in SBH.12 In particular, deficits in perceptual and motor timing13 and speech dysfluencies14 have been related to cerebellar dysmorphologies associated with spina bifida.
The purpose of our study was to explicitly acknowledge the specific contribution of the ACM in the cognitive profile of children with spina bifida. Because of the complex neuropathological conditions of patients with spina bifida mentioned above, the method applied was based on comparisons of cognitive profiles of our study group with those of patients with cerebellar damage and hydrocephalus found in the literature. Our premise is that tasks that rely on cognitive functions such as automation, verbal fluency and visual processing are subserved by cerebellar structures, and thus could be disrupted in SBH due to cerebellar malformation. For this, a complete battery of neuropsychological tests, including tests for cerebellar cognitive functions, was administered to two groups of children with spina bifida, those without ACM (ACM−) and those with ACM (ACM+).
Methods
Study group
The study group was selected by using the following inclusion criteria: (1) born with spina bifida in our hospital or admitted for surgery later on; (2) date of birth between 1 January 1988 and 31 December 1997; (3) T1‐weighted magnetic resonance imaging (MRI) conclusive for absence or presence of ACM. The diagnosis of this condition was based on the MRI criteria defined by Stevenson.1
Seventy eight children fulfilled these inclusion criteria. For various reasons (refusal to participate, foreign language, profound retardation precluding formal testing), 32 of the 78 selected patients could not be adequately examined. Thus, the final study group consisted of 46 children. All children underwent spinal surgery within 5 years after birth.
To assess the effect of ACM, subgroups of ACM− and ACM+ children were formed. Furthermore, non‐retarded ACM− and ACM+ groups were selected on the basis of a verbal IQ (VIQ) of ⩾75 on the Wechsler Intelligence Scale for Children—third edition (WISC‐III).15 In this way, the possible confounding effects of global cognitive impairment were excluded.
At the time of assessment, children were between 6 and 15 years of age. Mean age and age range of the subgroups are presented in table 1.
Table 1 Mean scores (standard deviations) of cognitive outcome.
| All ACM− | All ACM+ | F | p Value | VIQ⩾75 ACM− | VIQ⩾75 ACM+ | F | p Value | |
|---|---|---|---|---|---|---|---|---|
| n | 19 | 27 | 17 | 17 | ||||
| Age in years (range) | 10.0 (6.6–14.11) | 10.3 (6.4–15.1) | 9.7 (6.6–14.11) | 9.8 (6.4–12.9) | ||||
| VIQ | 95.8 (15.3) | 80.3 (17.4) | F(1,44) = 9.9 | 0.003 | 98.9 (12.9) | 91.3 (9.1) | F(1,32) = 4.0 | 0.055 |
| PIQ | 94.2 (16.0) | 68.2 (16.5) | F(1,44) = 28.5 | 0.000 | 97.5 (13.3) | 77.7 (11.9) | F(1,32) = 20.9 | 0.000 |
| TIQ | 94.5 (15.8) | 72.6 (16.6) | F(1,44) = 20.2 | 0.000 | 97.9 (12.7) | 83.5 (8.2) | F(1,32) = 15.5 | 0.000 |
| VIQ–PIQ | 1.6 (13.1) | 12.1 (11.14) | F(1,44) = 8.5 | 0.006 | 1.47 (13.9) | 13.6 (12.5) | F(1,32) = 7.2 | 0.012 |
A significance level of p<0.05 was used.
ACM, Arnold‐Chiari‐II malformation; PIQ, performance IQ; TIQ, total IQ; VIQ, verbal IQ.
The experimental procedures of our study were approved by the Committee on Research Involving Human Subjects (CMO Regio Arnhem‐Nijmegen, Nijmegen, The Netherlands) of the Radboud University Nijmegen Medical Centre. Written consent was obtained from the parents of each subject.
Procedure
All children underwent an individual neuropsychological assessment, two assessment sessions for most children. The order of the neuropsychological tests was fixed. The total duration of the psychological assessment varied between 3.5 and 5 h.
Measures
The neuropsychological assessment intended to cover the following cognitive functions: intelligence, visual analysis and synthesis, visuomotor functioning, (non‐)verbal memory, processing speed, verbal skills and verbal fluency. Subtests of three different batteries of tests were used (WISC‐III; Kaufman‐ABC (K‐ABC); and Revised Amsterdam Kinder Intelligentie Test (RAKIT)), along with Stroop Color–Word Test, Bourdon‐Vos Concentration Test, Beery's Visuomotor Integration and 15‐Words Test.16
For the Stroop Color–Word Test, the subtests of the RAKIT and the 15‐Words Test, the oldest children passed the limits of the age norm. In these cases, the highest age norms were applied.
Statistical analysis
Statistical analyses were carried out with SPSS V.12.0.1 for Windows. Analyses of variance (ANOVAs) were used to examine the differences in ACM− and ACM+ with regard to VIQ and performance IQ (PIQ), and also the discrepancy between both (VIQ–PIQ). Multivariate analyses of variance (MANOVAs) were conducted with the different subtests for each cognitive function as dependent variables and group (ACM− v ACM+) as an independent variable. The same analyses were then carried out for the non‐retarded groups (VIQ⩾75 ACM− v VIQ⩾75 ACM+). Each of the ANOVAs was considered significant when p<0.05. Because of the large number of comparisons in MANOVAs, the α significance level 0.05 was adjusted using the Bonferroni correction.
Results
Cognitive outcome
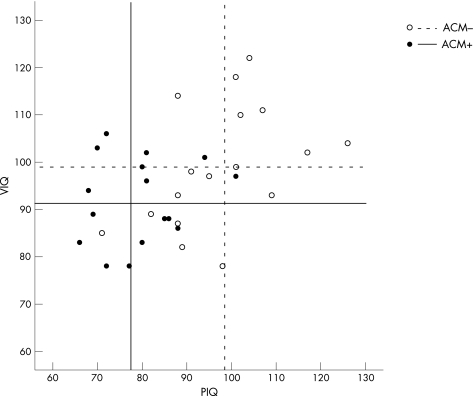
In the complete group, the factor ACM was significant: ACM+ children had a lower VIQ (fig 1, table 1) and TIQ than ACM− children. In the non‐retarded group, these differences were smaller and reached significance only for PIQ and TIQ; ACM− and ACM+ children matched well on VIQ. Overall, discrepancies between VIQ and PIQ were significant for both the complete and the non‐retarded groups.
Figure 1 Scatterplot of individual verbal IQ (VIQ) and performance IQ (PIQ) scores per group. Uninterrupted lines indicate the mean VIQ (horizontal line) and mean PIQ (vertical line) for the ACM+ group. Dotted lines display mean VIQ and PIQ of the ACM− group.
Cognitive profile
In the complete group, ACM+ children (all ACM+) were significantly impaired on all tests as compared with ACM− children (all ACM−), because of global cognitive impairment. Therefore, to assess differences in cognitive profile, we focused on a comparison of the non‐retarded ACM− with the non‐retarded ACM+ group (VIQ⩾75 ACM− v VIQ⩾75 ACM+).
The MANOVAs indicated significant main effects for visual analysis and synthesis, verbal memory and verbal fluency.
Table 2 gives the mean scores, standard deviations and p values of MANOVAs.
Table 2 Mean scores (standard deviations) of cognitive profile and results of the multivariate analyses of variance.
| Test battery | VIQ⩾75 ACM− | n | VIQ⩾75 ACM+ | n | F | p Value | |
|---|---|---|---|---|---|---|---|
| Visual analysis and synthesis | 17 | 17 | |||||
| Picture completion | WISC‐III | 10.7 (2.6) | 8.4 (2.5) | ||||
| Object assembly | WISC‐III | 9.2 (2.8) | 5.9 (3.1) | ||||
| Picture arrangement | WISC‐III | 9.8 (2.2) | 5.8 (2.1) | F(5,28) = 6.59 | 0.000 | ||
| Block design | WISC‐III | 9.5 (2.7) | 7.5 (2.6) | ||||
| Gestalt closure | K‐ABC | 10.1 (2.5) | 8.4 (3.8) | ||||
| Visuomotor function | 14 | 15 | |||||
| Mazes | WISC‐III | 10.0 (2.1) | 7.8 (3.6) | ||||
| VMI SS | Beery | 98.9 (8.7) | 90.1 (8.3) | F(3,25) = 4.43 | 0.012 | ||
| Discs | RAKIT | 15.9 (5.3) | 10.5 (5.4) | ||||
| Verbal memory | — | 17 | 16 | ||||
| 15‐Words test total | — | 46.2 (9.6) | 34.1 (12.7) | ||||
| 15‐Words test recall | — | 10.1 (2.8) | 6.1 (3.3) | F(4,28) = 5.67 | 0.002 | ||
| Word order | K‐ABC | 10.5 (2.2) | 7.8 (3.0) | ||||
| Digit span | WISC‐III | 10.4 (2.4) | 8.6 (2.0) | ||||
| Non‐verbal memory | — | 17 | 17 | ||||
| Hand movements | K‐ABC | 10.4 (2.2) | 9.8 (2.6) | F(2,31) = 1.12 | 0.34 | ||
| Spatial memory | K‐ABC | 9.8 (2.6) | 8.4 (2.9) | ||||
| Processing speed | 12 | 13 | |||||
| Coding | WISC‐III | 9.2 (2.2) | 6.5 (3.8) | ||||
| Symbol search | WISC‐III | 10.5 (2.8) | 7.4 (3.3) | F(4,20) = 1.73 | 0.182 | ||
| Stroop color* | — | 89.1 (22.0) | 99.0 (22.1) | ||||
| Bourdon RT tot* | — | 19.9 (5.2) | 21.7 (6.7) | ||||
| Verbal skills | 17 | 17 | |||||
| Similarities | WISC‐III | 10.4 (2.8) | 8.3 (2.3) | ||||
| Comprehension | WISC‐III | 9.5 (1.7) | 8.1 (2.2) | F(4,29) = 1.82 | 0.152 | ||
| Vocabulary | WISC‐III | 10.1 (2.9) | 8.9 (1.8) | ||||
| Arithmetic | WISC‐III | 9.7 (2.4) | 8.4 (3.4) | ||||
| Verbal fluency | RAKIT | 14.2 (4.7) | 17 | 8.5 (4.4) | 17 | F(1,32) = 12.8 | 0.001 |
ACM, Arnold‐Chiari‐II malformation; K‐ABC, Kaufman‐ABC; RAKIT, Revised Amsterdam Kinder Intelligentie Test; SS, sum of squares; VIQ, verbal IQ; VMI, visuomotor integration; WISC‐III, Wechsler Intelligence Scale for Children—third edition.
A significance level of p<0.05 was adjusted by using the Bonferroni correction.
*Score represents time to perform; so high scores indicate poor (slow) performance.
Discussion
The aim of our study was to delineate the specific influence of ACM in the cognitive profile of patients with spina bifida. Results showed that ACM+ patients had a significantly lower PIQ than ACM− patients, even after excluding the confounding effect of global cognitive impairment. In the non‐retarded group, the patients with and without ACM matched well on VIQ, which is not supposed to be associated with cerebellar dysfunction.
In terms of cognitive profile, the ACM+ group performed particularly poor on tests requiring visual analysis and synthesis, verbal memory, and verbal fluency. As all ACM+ patients also had hydrocephalus, which is inherent to myelomeningocoele, it is difficult to separate out the influences of hydrocephalus and ACM on cognitive impairment. Two methods, however, were applied to delineate the specific effect of the ACM. Firstly, the confounding effect of hydrocephalus was reduced by selecting non‐retarded ACM− and ACM+ groups matched on VIQ, leaving those patients who had a mild or well‐treated hydrocephalus. Secondly, the specific characteristics of the cognitive profile were compared with the neuropsychological literature on cerebellar disorders and hydrocephalus.
Recently, evidence has been presented that the cerebellum is part of a cerebrocerebellar network and contributes to motor processes and also to cognitive functions.17,18 Our study suggests the role of the cerebellum in both motor speed and coordination as well as higher cognitive functioning in patients with spina bifida. The non‐retarded ACM+ group showed impaired visual analysis and synthesis. On the one hand, considering the aspects of visuomotor integration of the tasks, low performances could be attributed to hydrocephalus.4,10 On the other hand, poor performances on these tasks could, in addition to hydrocephalus, reflect reduced processing speed and poor visual–spatial function related to cerebellar malformation.17,18,19,20
Furthermore, results showed deficits in verbal memory and fluency among ACM+ patients. This finding is consistent with the pattern of cognitive impairment reported in patients with cerebellar damage.18,21,20 Although deficits in verbal memory and verbal fluency have also been related to hydrocephalus in earlier studies,11,22,23 most of those studies are limited by a selection bias (inclusion of patients with hydrocephalus of heterogeneous aetiologies,9,11,23 or spina bifida of diverging severity6) or by an underestimation of the confounding influence of the ACM.6,11,22 Given the co‐occurrence of hydrocephalus and ACM in patients with spina bifida, we conclude that the deficits in verbal memory and fluency are most likely to be due to the cerebellar malformation.
To conclude, the current data support the hypothesis that hydrocephalus alone is not a sufficient explanation of the cognitive deficits in spina bifida. Impaired visual analysis and synthesis seem to be related to both hydrocephalus and ACM, whereas deficiencies in verbal memory and fluency may be attributed to ACM. As spina bifida is associated with complex neuropathology, further disentangling the contribution of the different additional malformations requires studies correlating anatomical neuroimaging data with cognitive measures.13
Acknowledgements
We thank all the parents and the children for their effort to take part in this study.
Abbreviations
ACM - Arnold‐Chiari malformation
ACM+ - patients with SB and ACM
ACM− - patients with SB but without ACM
ANOVA - analysis of variance
MANOVA - multivariate analysis of variance
PIQ - performance IQ
RAKIT - Revised Amsterdam Kinder Intelligentie Test
SBH - spina bifida and hydrocephalus
TIQ - total IQ
VIQ - verbal IQ
WISC‐III - Wechsler Intelligence Scale for Children—third edition
Footnotes
Competing interests: None declared.
This study was part of the Nijmegen Interdisciplinary Spina Bifida (NISB) research programme. This research programme is dedicated to fostering the care of children with spina bifida and their families.
The participating institutes and researchers are: Radboud University Medical Centre, Nijmegen: Department of Pediatric Neurology (N Geerdink; R Mullaart; J Rotteveel), Department of Medical Psychology/Pediatric Neurology (A Vinck; B Maassen), Department of Epidemiology and Biostatistics (N Roeleveld), Radboud University Nijmegen: Institute of Family and Child Care Studies (I Vermaes; J Gerris; J Janssens), Department of Empirical Theology (M van den Heuvel; H Schilderman; J van der Ven).
References
- 1.Stevenson K L. Chiari type II malformation: past, present, and future. Neurosurg Focus 2004161–7. [DOI] [PubMed] [Google Scholar]
- 2.Mirzai H, Ersahin Y, Mutluer S.et al Outcome of patients with meningomyelocele. Child's Nerv Syst 199814120–123. [DOI] [PubMed] [Google Scholar]
- 3.Soare P L, Ramondi A J. Intellectual and perceptual‐motor characteristics of treated myelomeningocele children. Am J Dis Child 1977131199–204. [DOI] [PubMed] [Google Scholar]
- 4.Wills K E. Neuropsychological functioning in children with spina bifida and/or hydrocephalus. J Clin Child Psychol 199322247–265. [Google Scholar]
- 5.Fletcher J M, Northrup H, Landry S H.et al Spina bifida: genes, brain, and development. Int Rev Res Ment Retard 20042963–117. [Google Scholar]
- 6.Barf H A, Verhoef M, Post M W M.et al Cognitive status of young adults with spina bifida. Dev Med Child Neurol 200345813–820. [DOI] [PubMed] [Google Scholar]
- 7.Heinsbergen I, Rotteveel J J, Roeleveld N.et al Outcome in shunted hydrocephalic children. Eur J Paediatr Neurol 2002699–107. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher J M, Bohan T P, Brandt M E.et al Cerebral white matter and cognition in hydrocephalic children. Arch Neurol 199249818–824. [DOI] [PubMed] [Google Scholar]
- 9.Brookshire B L, Fletcher J M, Bohan T P.et al Verbal and nonverbal skill discrepancies in children with hydrocephalus: a five‐year longitudinal follow‐up. J Pediatr Psychol 199520785–800. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher J M, Brookshire B L, Bohan T P.et al Early hydrocephalus. In: Rourke BP, ed. Nonverbal learning disability: the syndrome and the model. New York: Guilford Press, 1995
- 11.Iddon J L, Morgan D J, Loveday C.et al Neuropsychological profile of young adults with spina bifida with or without hydrocephalus. J Neurol Neurosurg Psychiatry 2004751112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis M, Hetherington R, Spiegler B J.et al Functional consequences of congenital cerebellar dysmorphologies and acquired cerebellar lesions of childhood. In: Broman SH, Fletcher JM, eds. The changing nervous system—neurobehavioural consequences of early brain disorder. New York: Oxford, 1999
- 13.Dennis M, Edelstein K, Hetherington R.et al Neurobiology of perceptual and motor timing in children with spina bifida in relation to cerebellar volume. Brain 20041271292–1301. [DOI] [PubMed] [Google Scholar]
- 14.Huber‐Okrainec J, Dennis M, Brettschneider J.et al Neuromotor speech deficits in children and adults with spina bifida and hydrocephalus. Brain Lang 200280592–602. [DOI] [PubMed] [Google Scholar]
- 15.Wechsler D.Wechsler intelligence scale for children (NL). London: The Psychological Corporation, 2002
- 16.Kievit Th, deWit J, Groenendaal J H A.et alHandboek psychodiagnostiek voor de hulpverlening aan kinderen. Maarssen: Elsevier, 1998
- 17.Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 200012193–198. [DOI] [PubMed] [Google Scholar]
- 18.Schmahmann J D, Sherman J C. The cerebellar cognitive affective syndrome. Brain 1998121561–579. [DOI] [PubMed] [Google Scholar]
- 19.Molinari M, Petrosini L, Misciagna S.et al Visuospatial abilities in cerebellar disorders. J Neurol Neurosurg Psychiatry 200475235–240. [PMC free article] [PubMed] [Google Scholar]
- 20.Levisohn L, Cronin‐Golomb A, Schmahmann J D. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 20001231041–1050. [DOI] [PubMed] [Google Scholar]
- 21.Leggio M G, Silveri M C, Petrosini L.et al Phonological grouping is specifically affected in cerebellar patients: a verbal fluency study. J Neurol Neurosurg Psychiatry 200069102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeates K O, Enrile B G, Loss N.et al Verbal learning and memory in children with myelomeningocele. J Pediatr Psychol 199520801–815. [DOI] [PubMed] [Google Scholar]
- 23.Scott M A, Fletcher J M, Brookshire B L.et al Memory functions in children with early hydrocephalus. Neuropsychology 199812578–589. [DOI] [PubMed] [Google Scholar]