Hyperkinetic disorders such as chorea, ballism and dystonia can be observed after lesions (usually small strokes) affecting the thalamus, basal ganglia and related circuits.1 Neural circuit models of basal ganglia circuitry suggest that these result from disinhibition of thalamo‐cortical projections because of a reduction in inhibitory output from the internal pallidal segment. Increased thalamo‐cortical drive may lead to an increase in the excitability of excitatory and inhibitory circuits of the frontal areas of the cortex,2,3 including M1, that may contribute to hyperkinesia.
Circuits of the human motor cortex can be activated non‐invasively with transcranial magnetic stimulation (TMS) of the brain,4 and because the excitability of neural circuits in the cerebral cortex is not static, repetitive TMS (rTMS) can produce changes in neurotransmission that outlast the period of stimulation.5,6,7
rTMS has been proposed as a therapeutic tool in several psychiatric and neurological disorders, and a recent review suggested that the best effects of therapeutic rTMS are seen when protocols that depress network excitability are used to treat disorders characterised by cortical hyperexcitability.8
A recent study showed that excitability of the motor cortex can be reduced for 30–60 min after application of a novel paradigm of rTMS termed continuous θ burst stimulation (cTBS).9,10 This protocol leads to a decrease in the excitability of excitatory and inhibitory cortical circuits that is long lasting. Thus, after TBS, there is a decrease in the amplitude of the corticospinal volleys evoked by transcranial stimulation,10 and the size of the resulting motor‐evoked potentials.9 In addition, a reduction in excitability of intracortical inhibitory circuits was shown by a decrease in short latency intracortical inhibition, a putative marker of γ aminobutyric acid transaminase‐A activity.9
The aim of this preliminary study was to investigate whether cTBS of the motor cortex could reduce involuntary movements in a patient with hemichorea‐ballism.
We hypothesised that the reduced excitability in excitatory and inhibitory circuits of the motor cortex that follows cTBS9 could normalise excitability of frontal areas and improve hyperkinesias.
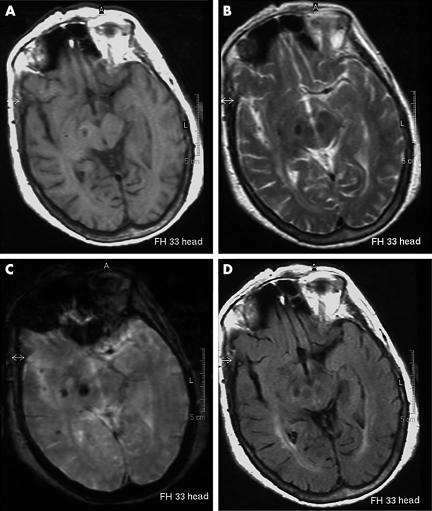
A 68‐year‐old woman with a history of hypertension and glaucoma suddenly presented with severe involuntary movements of the left arm, leg and face. An MRI of the brain, carried out a week after the stroke, showed a lesion with signal characteristics of subacute haemorrhage in the midbrain and the caudal diencephalon on the right side, and thus conceivably affecting the subthalamic nucleus or the substantia nigra, or both these structures (fig 1). A lesion of these structures would remove excitatory drive to the internal segment of the globus pallidus and the resulting decrease in pallidal inhibition of thalamus would lead to a consequent increase in thalamo‐cortical excitation. Treatment with levetiracetam (500 mg twice daily) produced no benefit.
Figure 1 MRI of the brain of the patient. (A) Axial SE T1‐weighted image (TR 582/TE 15). (B) Axial TSE image (TR 2200/TE 120). (C) Axial GE T2‐weighted image (TR 600/TE 23). (D) Axial T2‐FLAIR image (TR 6000/TE 100/TI 2000). The images show a subacute haemorrhagic lesion in the midbrain of the right side.
In the weeks after the stroke, there was mild improvement in the severity of dyskinesias. Movements on the left, and particularly in the arm, however, never resolved completely, and caused pain in the left shoulder.
Ten weeks after the stroke, neurological examination showed left hemichorea, particularly affecting the arm, with ballistic movements at the shoulder and distal choreo‐athetoid movements. Mild choreatic movements were also observed in the left hemi‐facial muscles and leg. Posture, voluntary movements or tasks requiring concentration increased intermittent dyskinesias at rest. The rest of the neurological examination was unremarkable apart from blindness of the left eye secondary to glaucoma. Treatment with increasing doses of tetrabenazine up to a dose of 25 mg thrice daily failed to produce any benefit after 2 months. At this time, rTMS of the motor cortex was carried out as an add‐on treatment.
The patient gave informed consent to the study that was performed with the approval of the appropriate Institutional ethics committee.
rTMS was applied over the left‐hand motor area by using a MagPro (Medtronic A/S, Copenhagen, Denmark) stimulator and a figure of eight‐shaped coil. The stimulation intensity was 80% of the active motor threshold (AMT), defined as the minimum single pulse intensity required to produce a motor evoked potential >200 μV on more than five out of ten trials from the contracted contralateral first dorsal interosseous.
rTMS was carried out using the cTBS pattern in which three pulses of stimulation are given at 50 Hz and repeated every 200 ms, for a total of 600 pulses.9 This protocol leads to pronounced and prolonged suppression of cortical excitability that reaches a maximum about 5–10 min after the end of the protocol.9
Sham rTMS was performed by using the same stimulator connected to the placebo butterfly coil MCF‐P‐B‐65, which has no stimulating effect on the cortex but produces auditory and tactile sensations similar to those produced by the real coil. The site of the stimulation and the number of stimuli were identical to those used for the real magnetic rTMS.
Two sessions of real rTMS (the first and the third) and one session of sham rTMS were carried out at intervals of about 10 days. At each session, the patient was evaluated by a skilled movement disorders neurologist. Both the patient and the neurologist who scored the dyskinesias were blinded to the rTMS protocol (sham or real) used in each session. Dyskinesias were measured before and 10 min after stimulation by means of a Dyskinesias Scale (ranging from 0 to 4, see appendix) scored in all the following conditions: at rest; while performing a distracting mental activity; while walking; and while performing voluntary tasks (pouring water, drinking from a glass, putting on a lab coat, buttoning and finger‐to‐nose manoeuvre). Drinking was the action on which the worst performance occurred and it was therefore the task chosen to compare the severity of dyskinesias in all the six assessments.
The evaluation of the involuntary movements was carried out live and video recordings of the first and second sessions were obtained.
The patient could not distinguish between real and sham stimulation.
Real rTMS produced a dramatic improvement in dyskinesias during functional tasks requiring the use of the upper limb (for video A and table, see http://jnnp.bmjjournals.com/supplemental).
Scores on the evaluation scale improved from 4 to 1 (pouring, drinking), from 3 to 2 (buttoning), from 3 to 1 (rest, finger‐to‐nose) and from 2 to 1 (walking) after the first session and similarly after the third session (table). The patient reported that the benefit lasted about 24 h after both sessions, with associated reduction of the pain at the left shoulder. After the sham stimulation (second session), no consistent variations were reported by the patient or by the evaluating neurologist. Basal scores on that day were 3 for pouring, drinking, buttoning and finger‐to‐nose manoeuvre and 2 for rest and walking: a slight improvement was noticed by the evaluating neurologist but was judged to be insufficient to modify the rating on each of the considered tasks (for video B and table, see http://jnnp.bmjjournals.com/supplemental).
Studies in patients with chorea due to Huntington's disease3 and in a patient with diabetes with hemiballism–hemichorea2 suggest that in these conditions increased thalamo‐cortical drive may increase excitability of excitatory and inhibitory circuits of frontal areas of the cortex, including M1, and contribute to the development of hyperkinesias. The present study shows that rTMS protocols that suppress the excitability of excitatory and inhibitory circuits of the motor cortex may be useful in the treatment of hyperkinetic disorders. This would be consistent with a recent study in which low‐frequency rTMS, which is another way of decreasing cortical excitability, was used on midline frontal cortex to reduce dyskinesias in patients with Huntington's disease.11
In this study we cannot determine whether the principal effect of cTBS occurs through its suppression of excitatory or inhibitory circuits, or both. What is more certain, however, is that the effects are likely to have occurred because of a change in cortical rather than subcortical circuits. Thus, cTBS is known to suppress excitatory circuits that generate I waves4 within the motor cortex. Similarly, cTBS reduces short‐latency intracortical inhibition,9 which again is a cortical phenomenon.4 Currently, there is no evidence that cTBS leads to any changes in excitability of subcortical circuits.
Because the effects of TMS are short‐lived, cTBS is not suitable as a chronic intervention. It can, however, turn out to be useful in selecting patients for implantation with cortical epidural electrodes as both TMS and epidural stimulation appear to activate similar elements in the motor cortex.12 A similar approach has been suggested by Lefaucheur13 to select patients with pain who may be candidates for chronic epidural motor cortex stimulation.
An additional table and videos 1 and 2 are available at http://jnnp.bmjjournals.com/supplemental
Supplementary Material
Footnotes
Competing interests: None declared.
Informed consent was obtained for publication of the patient's details in this report.
An additional table and videos 1 and 2 are available at http://jnnp.bmjjournals.com/supplemental
References
- 1.Lee M S, Marsden C D. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord 19949493–507. [DOI] [PubMed] [Google Scholar]
- 2.Ziemann U, Koc J, Reimers C D.et al Exploration of motor cortex excitability in a diabetic patient with hemiballism‐hemichorea. Mov Disord 2000151000–1005. [DOI] [PubMed] [Google Scholar]
- 3.Modugno N, Curra A, Giovannelli M.et al The prolonged cortical silent period in patients with Huntington's disease. Clin Neurophysiol 20011121470–1474. [DOI] [PubMed] [Google Scholar]
- 4.Di Lazzaro V, Oliviero A, Pilato F.et al The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 2004115255–266. [DOI] [PubMed] [Google Scholar]
- 5.Berardelli A, Inghilleri M, Rothwell J C.et al Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res 199812279–84. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Classen J, Gerloff C.et al Depression of motor cortex excitability by low‐frequency transcranial magnetic stimulation. Neurology 1997481398–1403. [DOI] [PubMed] [Google Scholar]
- 7.Pascual Leone A, Valls‐Solè J, Wassermann E M.et al Responses to rapid‐rate transcranial magnetic stimulation of the human motor cortex. Brain 1994117847–858. [DOI] [PubMed] [Google Scholar]
- 8.Wassermann E M, Lisanby S H. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol 20011121367–1377. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y Z, Edwards M J, Rounis E.et al Theta burst stimulation of the human motor cortex. Neuron 200545201–206. [DOI] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Pilato F, Saturno E.et al Theta‐burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol 2005565945–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brusa L, Versace V, Koch G.et al Improvement of choreic movements by 1 Hz repetitive transcranial magnetic stimulation in Huntington's disease patients. Ann Neurol 200558655–656. [DOI] [PubMed] [Google Scholar]
- 12.Di Lazzaro V, Oliviero A, Pilato F.et al Comparison of descending volleys evoked by transcranial and epidural motor cortex stimulation in a conscious patient with bulbar pain. Clin Neurophysiol 2004115834–838. [DOI] [PubMed] [Google Scholar]
- 13.Lefaucheur J P. Transcranial magnetic stimulation in the management of pain. Suppl Clin Neurophysiol 200457737–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.