Abstract
Background
The two most common types of acquired reading disorder resulting from damage to the territory of the dominant posterior cerebral artery are hemianopic and pure alexia. Patients with pronounced hemianopic alexia have a right homonymous hemianopia that encroaches into central or parafoveal vision; they read individual words well, but generate inefficient reading saccades when reading along a line of text. Patients with pure alexia also often have a hemianopia but are more disabled, making frequent errors on individual words; they have sustained damage to a brain region that supports efficient word identification.
Objective
To investigate the differences in lesion site between hemianopic alexia and pure alexia groups, as rehabilitative techniques differ between the two conditions.
Methods
High‐resolution magnetic resonance images were obtained from seven patients with hemianopic alexia and from six patients with pure alexia caused by a left occipital stroke. The boundary of each lesion was defined and lesion volumes were then transformed into a standard stereotactic space so that regional comparisons could be made.
Results
The two patient groups did not differ in terms of damage to the medial left occipital lobe, but those with pure alexia had additional lateral damage to the posterior fusiform gyrus and adjacent tissue.
Conclusions
Clinicians will be able to predict the type of reading disorder patients with left occipital lesions have from simple tests of reading speed and the distribution of damage to the left occipital lobe on brain imaging. This information will aid management decisions, including recommendations for reading rehabilitation.
Although an acquired reading disorder (alexia) is usually part of a more generalised language disorder (aphasia), it can occur as an isolated deficit, usually as a consequence of damage to the brain within the distribution of the left posterior cerebral artery. The two common forms of isolated alexia are hemianopic alexia and pure alexia. The first is the result of a right homonymous hemianopia (RHH), which impairs text reading more than single‐word reading. This is because, in left‐to‐right readers, visual information to the right of fixation is needed to plan rightward reading saccades.1,2,3 Scanning along a line of text is affected if the RHH encroaches to within 5° of fixation, in right foveal or parafoveal vision.4 However, the relationship between text‐reading speed and the number of degrees of sparing of central vision is not linear, and most symptomatic patients have defects encroaching to within 2–3° of fixation or less. By contrast, patients with pure alexia have a severe impairment of single‐word recognition. Although they often have an associated RHH, their syndrome is not a consequence of this deficit, and there have been a few patients with pure alexia without an accompanying field defect.1 Rather, their impairment is the consequence of damage to a whole‐word recognition system that allows a skilled reader to recognise seven‐letter words as quickly as words of three letters. Patients with pure alexia have damage in the whole‐word recognition system, its connections to primary visual areas or, occasionally, its connections to “higher” language areas. Although these patients can still read, they rely on a more‐or‐less intact letter recognition system and a laborious reversed‐spelling procedure, covert or overt, to arrive at the word's identity, so‐called “letter‐by‐letter reading”. Thus, the word “dog” is not recognised, but explicitly spelling out “d”, “o”, “g” permits essential reading.
It is important to differentiate between the two conditions, as there is specific rehabilitation for hemianopic alexia.5 Although occasionally some success has been reported in retraining patients with pure alexia,6 most regard pure alexia as an irremediable condition. Pure alexia is more disabling, but many patients with hemianopic alexia find text reading so laborious that they give up recreational reading, and if reading speed is an important skill in their job, their continuing employment may be at risk.
The aim of this study was to determine whether pure alexia and hemianopic alexia can be differentiated by the limits of their left occipital lesion on magnetic resonance images (MRIs). Although much has been written about the pathological anatomy of pure alexia,7,8,9,10 this is the first study directly comparing lesion site and size between patients with pure alexia and hemianopic alexia. The outcome is practical, in terms of diagnosis and referral for appropriate rehabilitation. Further, it provides additional data that identify the region responsible for rapid whole‐word recognition, by excluding areas that may be damaged by posterior cerebral artery (PCA) territory stroke but do not contribute to the syndrome of pure alexia.
Methods
We selected our 13 patients from a larger group being studied in a rehabilitation programme. We selected those at the behavioural extremes between pure alexia and hemianopic alexia. Those with pure alexia (n = 6) had a prominent word length effect (WLE)—that is, the speed of single‐word reading was linearly related to the number of letters in a word. Slow single‐word reading resulted in slow text reading. Patients with hemianopic alexia (n = 7) had a much smaller WLE, but were impaired in text reading compared with normal subjects as judged by the Neale analysis of reading.11 All the patients were right handed. Most of them had focal damage due to infarction (n = 11, including all seven of the hemianopic alexia group). Three patients of the pure alexia group had a more unusual cause of their stroke: two of them had had an intracerebral haemorrhage, one of which was secondary to a head injury and the other was thought to be spontaneous, and the third patient sustained venous infarction secondary to cortical venous sinus thrombosis. One patient of the hemianopic alexia group was female, as were two patients of the pure alexia group; three patients of the hemianopic alexia group had macular splitting hemianopia or quadrantanopia, as did four patients of the pure alexia group.
T1‐weighted MRIs of the brain were acquired for each patient in 180 axial planes with 1‐mm3 isotropic voxels. The images were manipulated using both MRIcro12 software and Analyze (Biomedical Imaging Resource Mayo Foundation, Jacksonville, Florida, USA). The analysis was carried out by APL, who at the time was unaware of the clinical details and psychological profiles of the patients. Each brain image was inspected in the three orthogonal planes, oriented around the x, y and z axes. Firstly, the stroke margins were outlined by direct inspection. The volume of the stroke was used to create a “strokemask”, to protect the whole‐brain normalisation process from undue warping caused by cost‐function effects when damaged brains are matched to normal templates.13 Normalisation of each brain in standard stereotactic space was carried out using statistical parametric mapping (SPM99) (Wellcome Department of Cognitive Neurology, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm/softwarespm99). The parameters from each whole‐brain normalisation process were then applied to the stroke volume from that brain to transform it into the conventional stereotactic space devised by the Montreal Neurological Institute, Montreal, Canada.14 The normalised brain images were visually inspected for goodness of fit to the SPM99 T1‐weighted canonical MRI template. Six of the 13 scans needed some manual adjustment (mainly in the z plane, and always an upward shift) of between 4 and 8 mm (2–4 voxels), as one of the chronic effects of the loss of volume due to the stroke was to cause a ventral shift in the residual left occipital brain tissue.
Once anatomical normalisation had been completed for each stroke volume, comparisons could be made across patients and across groups. Normalised stroke volume images were imported back into MRIcro. Images from each group (pure alexia and hemianopic alexia) were then overlaid on the SPM canonical T1‐weighted template. This programme colour codes each voxel within the left occipital lobe to display the number of patients in a group whose lesion encompassed that voxel within their lesion.
Results
Demographic data
No significant differences were found between the two groups in terms of age (hemianopic alexia median 68 years and interquartile range {10}, pure alexia median 63 years and interquartile range {26}, Mann–Whitney two‐tailed test p = 0.5) or time since stroke (hemianopic alexia 27 months and {56}, pure alexia 49 months and {33}, p = 0.4). The pure alexia group had roughly twice the volume of infarction compared with the hemianopic alexia group, as indexed by the total number of voxels in each strokemask (hemianopic alexia 3204 and {2100}, pure alexia 5480 and {4564}, p = 0.022). Despite the differences in infarct size, there were no significant differences between the groups on the following neuropsychological test batteries: a modified IQ test (Wechsler Abbreviated Scale of Intelligence15: hemianopic alexia 114 and {15}, pure alexia 96 and {15}, p = 0.115); a test of visuospatial abilities (cube analysis subtest of the visual object and space perception battery16: hemianopic alexia 9/10 and {2}, pure alexia 9/10 and {2.5}, p = 0.836); recognition memory test for words17: (hemianopic alexia 43/50 and {9}, pure alexia 41/50 and {10}, p = 0.731).
Behavioural data
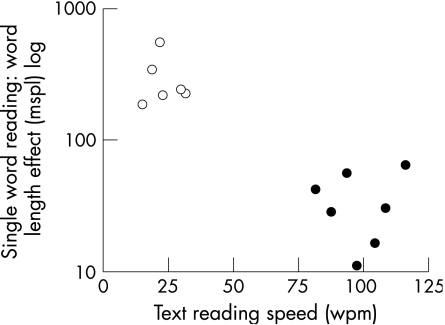
The hemianopic alexia group was significantly quicker at reading text (hemianopic alexia 98 words per minute (wpm) and {21}, pure alexia 23 wpm and {12}, p = 0.001), and single three‐letter words (hemianopic alexia 830 ms and {254}, pure alexia 1495 ms and {925}, p = 0.001) than the pure alexia group (fig 1). We arbitrarily chose a behavioural cut‐off of a WLE = 100 ms/letter.
Figure 1 Single‐word reading speed as a function of word length, measured as milliseconds per letter (mspl), plotted against text reading speed, in words per minute (wpm), for the pure alexia (PA) group (open circles) and the hemianopic alexia group (closed). The y axis is a log scale, as some patients with PA were very slow at single‐word reading. The arbitrary cut‐off of 100 mspl, used to distinguish between the two groups, is indicated. The upper limit of normal for single‐word reading is 10 mspl. The lower limit of normal for text reading is 112 wpm.
Image data
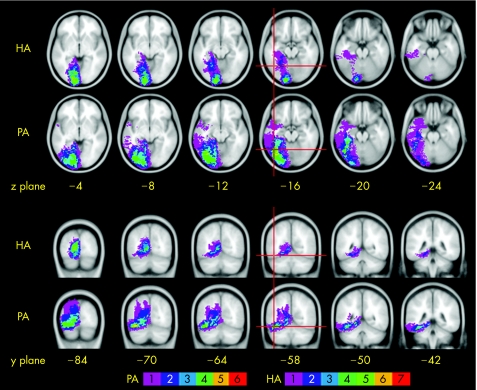
As shown in fig 2, the common focus of lesion site in the hemianopic alexia group was within the medial left occipital lobe, centred on the calcarine sulcus (the location of primary visual cortex) and often affecting the lingual gyrus below. In some patients, the area of damage extended in both anterior and ventral directions, and into more anterior medial temporal structures including parts of the lingual, parahippocampal and hippocampal gyri. The pure alexia group showed a similar medial occipital lesion overlap, accounting for their right visual field defects, with extension in some patients into the medial temporal lobe. In addition, the pure alexia group had damage extending into the lateral occipito‐temporal junction, a site undamaged in the hemianopic alexia group. This location was in the posterior part of the fusiform gyrus. Four of the six patients with pure alexia had damage to the voxel occupying the stereotactic coordinate identified as the centre of the visual word form area (VWFA), a brain region that supports rapid and efficient word recognition,18 and the other two patients had damage within 4 mm of this coordinate (indicated by cross‐hairs in fig 2).
Figure 2 Lesion overlap maps in the axial (top two rows) and coronal (bottom two rows) planes. The anatomically normalised lesions are overlaid on a standard magnetic resonance image template, the mean from 152 normal subjects normalised into standard stereotactic space, available in SPM99. The template is orientated around the plane of the anterior and posterior commissures. The distance on the axial images is in millimetres above (positive) and below (negative) this plane, and that in the coronal images is the distance behind the anterior commissure. The voxels are colour coded to indicate how many patients had that region included within their lesion (see scale). The red cross‐hairs indicate the voxel at the centre of the so‐called “visual word form area” identified from a meta‐analysis of 27 functional imaging studies of reading in normal subjects (coordinates in standard stereotactic space: x = −44, y = −58, z = −15).18. HA, hemianopic alexia; PA, pure alexia.
Discussion
Dejerine19 was the first to attempt an anatomical description of pure alexia. He proposed that a specialised region of the brain associated with storing the visual memory of letters had become disconnected from the primary visual cortex. He reasoned that this specialised region was separate from other language areas, as his patient with pure alexia could speak, comprehend and write normally. He thought that this region was located in the left angular gyrus. Over time his concept has remained largely intact although it has been revised.20 The six patients with pure alexia in this study were relatively unimpaired on letter recognition, but all had severely impaired whole‐word recognition. Recent work has identified a VWFA to be located within the left occipito‐temporal sulcus, between the posterior left fusiform and inferior temporal gyri.7,9,21 The syndrome of pure alexia is attributed to damage to this region or its connections. All the six patients with pure alexia had damage to the voxel occupying the stereotactic coordinate identified as the centre of the VWFA,18 or voxels within 4 mm of this coordinate.
Others have conducted lesion correlation studies to identify the critical regions associated with pure alexia, but they have either not used a patient control group8,9,10 or have used x ray computed tomography scanning, with lower spatial resolution.7 Having a control group is important to exclude “bystander” regions, included in the volume of damage but not contributing to the syndrome of pure alexia. The lesion overlap map produced by Cohen et al,8 using similar techniques to those described here, identified the greatest overlap of lesion site in the medial left occipital lobe, as does the computed tomogram overlap map of Damasio and Damasio.9 The study by De Renzi et al,10 which also did not include a control group, identified a brain slice that included the primary visual cortex as the one most commonly associated area with pure alexia. These findings are not surprising as hemianopia is the most commonly associated—but not causative—deficit in patients with pure alexia.9 Binder and Mohr7 used a hemianopic control group in their study, although these patients were reported as having no reading disorder. Their subtraction maps are similar to those presented here, but are technically inferior given the lower spatial resolution of computed tomography scanning (slice thickness 7 mm compared with 1 mm).
The reason why patients with hemianopic alexia and pure alexia share a partial lesion overlap is because the most common cause of both these syndromes is the same: thrombo‐embolic stroke. The PCA has four main cortical branches. The first two, the anterior and posterior temporal arteries, supply the ventral aspect of the lingual gyri, much of the parahippocampal and hippocampal gyri, and crucially, the fusiform gyrus. These branches leave the PCA at an almost 90° angle. The remaining artery continues as the calcarine branch, which gives off the parieto‐occipital artery before terminating in the occipital substance.22,23 This vascular arrangement partly explains why it is so rare to find patients with pure alexia who do not have a hemianopia. Small emboli passing down the PCA are more likely to spare the temporal branches (and thus the fusiform gyrus) and lodge in the calcarine or the parieto‐occipital branch causing a hemianopia. A larger, proximal embolism is more likely to affect all four branches, causing infarction of both the medial occipital and the occipito‐temporal cortex. A further important anatomical factor that will determine the lesion site and volume is the degree of collateral supply to occipito‐temporal regions from pial branches of the middle cerebral artery; this probably varies considerably across patients and could explain why some PCA infarcts affect only medial temporal regions whereas others spread more anteriorly and laterally.
We selected extreme exemplars of pure alexia and hemianopic alexia from our group of patients for this investigation to give us the best chance of isolating important anatomical differences between the two patient groups. The syndromes of pure alexia and hemianopic alexia probably form a continuum, and patients with aspects of both syndromes will have an intermediate lesion profile, which itself will depend on the mechanism of cerebral insult and, if vascular, the vagaries of individual cortical blood supply. Nevertheless, our results will allow clinicians to predict whether a patient is most likely to have pure alexia or hemianopic alexia, depending on their reading speeds, perimetry and lesion profile. In particular, infarction or haemorrhage that involves the posterior left fusiform gyrus and adjacent tissue predicts that the patient is unlikely to benefit from rehabilitation techniques designed for hemianopic alexia as these focus on either visual field restoration or oculo‐motor rehabilitation, rather than attempting to improve word‐form identification. This will facilitate patient selection for specific rehabilitation programmes.
Acknowledgements
This work was supported by a grant from The Wellcome Trust, which had no role in the study design or analysis.
Abbreviations
MRI - magnetic resonance image
PCA - posterior cerebral artery
RHH - right homonymous hemianopia
SPM - statistical parametric mapping
VWFA - visual word form area
WLE - word length effect
wpm - words per minute
Footnotes
Competing interests: None.
Ethical approval: This study was approved by the local research ethics committee.
Informed consent was obtained from all the patients for being studied.
References
- 1.Leff A P, Crewes H, Plant G T.et al The functional anatomy of single‐word reading in patients with hemianopic and pure alexia. Brain 2001124510–521. [DOI] [PubMed] [Google Scholar]
- 2.Leff A P, Scott S K, Crewes H.et al Impaired reading in patients with right hemianopia. Ann Neurol 200047171–178. [PubMed] [Google Scholar]
- 3.Zihl J. Eye movement patterns in hemianopic dyslexia. Brain 1995118891–912. [DOI] [PubMed] [Google Scholar]
- 4.Rayner K. Eye movements in reading and information processing: 20 years of research. Psychol Bull 1998124372–422. [DOI] [PubMed] [Google Scholar]
- 5.Kerkhoff G, Munsinger U, Eberle‐Strauss G.et al Rehabilitation of hemianopic alexia in patients with postgeniculate visual field disorders. Neuropsychol Rehabil 1992221–41. [Google Scholar]
- 6.Maher L M, Clayton M C, Barrett A M.et al Rehabilitation of a case of pure alexia: exploiting residual abilities. J Int Neuropsychol Soc 19984636–647. [DOI] [PubMed] [Google Scholar]
- 7.Binder J R, Mohr J P. Topography of callosal reading pathways. Brain 19921151807–1826. [DOI] [PubMed] [Google Scholar]
- 8.Cohen L, Martinaud O, Lemer C.et al Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex 2003131313–1333. [DOI] [PubMed] [Google Scholar]
- 9.Damasio A R, Damasio H. The anatomic basis of pure alexia. Neurology 1983331573–1583. [DOI] [PubMed] [Google Scholar]
- 10.De Renzi E, Zambolin A, Crisi G. The pattern of neuropsychological impairment associated with left posterior cerebral artery infarcts. Brain 19871101099–1116. [DOI] [PubMed] [Google Scholar]
- 11.Neale M D.Neale analysis of reading ability. Oxford, UK: NFER‐Nelson, 1989
- 12.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 200012191–200. [DOI] [PubMed] [Google Scholar]
- 13.Brett M, Leff A P, Rorden C.et al Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 200114486–500. [DOI] [PubMed] [Google Scholar]
- 14.Evans A C, Collins D L, Mills S R.et al 3D statistical neuroanatomical models from 305 MRI volumes. Proc IEEE Nucl Sci Symp Med Imaging Conf 19931813–1817.
- 15.Anon The Psychological Corporation Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio: Harcourt Brace & Company, 1999
- 16.Warrington E K, James M.The visual object and space perception battery. Bury St Edmunds: Thames Valley Test Company, 1991
- 17.Warrington E K.Recognition memory test. Windsor: NFER Nelson, 1984
- 18.Jobard G, Crivello F, Tzourio‐Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage 200320693–712. [DOI] [PubMed] [Google Scholar]
- 19.Dejerine J. Contribution a l'étude anatomo‐pathologique et clinique des differentes variétés de cécité‐verbale. Memoires Soc Biol 1892461–90. [Google Scholar]
- 20.Geschwind N. Disconnexion syndromes in animals and man. I. Brain 196588237–294. [DOI] [PubMed] [Google Scholar]
- 21.Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage 200422466–476. [DOI] [PubMed] [Google Scholar]
- 22.Salamon G.Atlas de la vascularisation arterielle du cerveau chez l'homme. Paris: Sandoz, 1973
- 23.Smith C G, Richardson W F. The course and distribution of the arteries supplying the visual (striate) cortex. Am J Ophthalmol 1966611391–1396. [DOI] [PubMed] [Google Scholar]