Abstract
Background
Some ganglioside complexes (GSCs) are target antigens for serum antibodies in patients with Guillain–Barré syndrome (GBS). Anti‐GSC antibodies may be associated with particular clinical features of GBS.
Objective
To investigate antibodies to GSCs in the sera of patients with Miller Fisher syndrome (MFS) characterised by elevation of the IgG anti‐GQ1b antibody.
Results
In all, 7 of 12 (58%) consecutive patients with MFS were found to have IgG antibodies to GSCs containing GQ1b, of whom 5 had IgG antibodies to GQ1b‐GM1 complex (GQ1b/GM1) and 2 had antibodies to GQ1b/GD1a; 4 of 5 patients without sensory symptoms had anti‐GQ1b/GM1 antibodies.
Conclusions
At least three different specificities in MFS‐associated antibodies, GQ1b‐specific, anti‐GQ1b/GM1‐positive and anti‐GQ1b/GD1a‐positive, were observed. In patients with MFS not only GQ1b itself but also clustered epitopes of GSCs, including GQ1b, may be considered to be prime target antigens for serum antibodies. A tendency to escape sensory disturbances is shown by anti‐GQ1b/GM1‐positive MFS.
We recently reported that some ganglioside complexes (GSCs) are target antigens for serum antibodies in patients with Guillain–Barré syndrome (GBS), an acute immune‐mediated polyradiculoneuropathy, and suggested that anti‐GSC antibodies may be associated with particular clinical features of GBS.1 Because glycolipids including gangliosides tend to form clustered complexes with cholesterols in lipid rafts in the plasma membrane,2 anti‐GSC antibodies are likely to cause nerve dysfunction through binding to GSCs in lipid rafts in neuronal membranes.
Miller Fisher syndrome (MFS) is characterised by a clinical triad of ophthalmoplegia, ataxia and areflexia, and is considered to be a variant of GBS.3 The presence of the IgG anti‐GQ1b antibody in serum is an excellent diagnostic marker for MFS.4 This antibody often cross reacts with GT1a4,5 and is pathophysiologically associated with ophthalmoplegia or ataxia in MFS and GBS.5,6,7 Thus, MFS is a clinically and serologically well‐defined syndrome with a pathophysiological mechanism similar to that of GBS, which suggests that patients with MFS may also have anti‐GSC antibodies. Here, we examined the serum samples of patients with MFS and found antibodies specific for a mixture of two gangliosides, including GQ1b or GT1a.
Methods
ELISA for anti‐GSC antibodies in serum from patients with MFS
Antibodies to GSC were investigated in acute‐phase serum samples collected from consecutive patients with MFS, who were diagnosed at the National Defense Medical College hospital, Saitama‐Ken, Japan, between April 1994 and December 2004. The diagnosis of MFS was based on acute self‐limited ophthalmoplegia, ataxia and areflexia without marked limb weakness, the involvement of CNS or other neurological diseases. The ELISA was carried out for antibodies to the gangliosides GM1, GM2, GD1a, GD1b, GT1a, GT1b and GQ1b, as described previously.8,9 When the corrected optical density was >0.1, the serum was considered to be positive. The ELISA for anti‐GSC antibodies was carried out as described in our previous report.1 GSCs used in the ELISA contained two of the above seven ganglioside antigens. Gangliosides were mixed for 30 min before their application to the ELISA. Anti‐GSC antibody‐positive samples were overlaid for thin‐layer chromatography immunostaining, as described previously,1 and the clinical features of anti‐GSC antibody‐positive patients with MFS were analysed. The above procedures were carried out at room temperature.
Immunoabsorption of anti‐GSC antibody‐positive serum samples
Anti‐GSC antibodies were absorbed in antigen‐coated ELISA wells, as described previously.5,9 Ganglioside antigens used for the absorption test were GSCs, a mixture of two gangliosides (250 ng each) or 500 ng of each ganglioside. Uncoated wells were used as controls. Anti‐GSC antibody‐positive serum diluted 1:40 with 1% bovine serum albumin in phosphate‐buffered saline was used, and the residual activities of the supernatants on the GSCs were estimated with ELISA. The percentage absorption of anti‐GSC antibody activity was calculated as described previously.9
Results
Anti‐ganglioside antibody assay and representative serum data
Acute‐phase serum samples were collected from 12 patients with MFS, 10 (83%) of whom had IgG anti‐GQ1b antibodies. The results from the ELISA showed that 7 of the 12 (58%) patients had serum antibodies to GSCs, such as GQ1b/GM1, GQ1b/GD1b, GQ1b/GD1a, GT1a/GM1, GT1a/GD1b, GT1a/GD1a and GQ1b/GT1b (table 1), but not to GSCs without GQ1b or GT1a. Antibodies to GQ1b/GM1, GT1a/GM1 and GT1a/GD1b were frequent. One patient (patient 7) had no anti‐GQ1b or anti‐GT1a antibodies, but had antibodies to GQ1b/GM1 and GT1a/GM1. In contrast with anti‐GSC antibodies in GBS, no antibodies to the GSCs consisting of two of the four major gangliosides, GM1, GD1a, GD1b and GT1b, were found in patients with MFS.
Table 1 Anti‐ganglioside complex antibodies in 12 consecutive patients with Miller Fisher syndrome.
| Corrected OD | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | Age (year) | Sex | Involved cranial nerves* | Sensory signs† | Anti‐GQ1b | Anti‐GT1a | Anti‐GQ1b/GM1 | Anti‐GQ1b/GD1b | Anti‐GT1a/GM1 | Anti‐GT1a/GD1b | Anti‐GQ1b/GD1a | Anti‐GT1a/GD1a | Anti‐GQ1b/GT1b | Other anti‐ glycolipid antibodies |
| 1 | 32 | M | + | 0.13 | 0.72 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | ||
| 2 | 65 | F | 7 | + | 0.12 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | |
| 3 | 38 | M | + | 0.86 | 0.55 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | ||
| 4 | 68 | F | ++‡ | 0.85 | 0.74 | (−) | (−) | (−) | (−) | (−) | (−) | (−) | ||
| 5 | 27 | M | + | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | ||
| 6 | 56 | F | + | 0.55 | (−) | 0.79 | (−) | 0.28 | 0.10 | (−) | (−) | (−) | ||
| 7 | 12 | F | − | (−) | (−) | 0.11 | (−) | 0.55 | (−) | (−) | (−) | (−) | ||
| 8 | 64 | M | 7 | − | 0.42 | (−) | 0.67 | (−) | 0.70 | 0.39 | (−) | (−) | (−) | |
| 9 | 69 | M | − | 0.77 | (−) | 1.08 | (−) | 1.02 | 0.56 | (−) | (−) | (−) | ||
| 10 | 38 | F | − | 0.11 | (−) | 0.70 | 0.63 | 0.84 | 0.60 | (−) | (−) | (−) | ||
| 11 | 35 | M | ++‡ | 0.20 | (−) | (−) | (−) | (−) | (−) | 0.40 | 0.22 | 0.42 | ||
| 12 | 52 | F | 9, 10 | + | 1.28 | 1.76 | (−) | (−) | (−) | (−) | 1.56 | (−) | 1.68 | IgM: GM1 |
OD, optical density; (−), antibody negative.
ELISAs were repeated twice, and the mean OD of the two experiments was calculated.
*Affected cranial nerves other than 3, 4, and 6. Because of the lack of anti‐GT1a and GD1b in patient 6 and anti‐GQ1b and GM1 in patient 7, anti‐GT1a/GD1b and anti‐GQ1b/GM1 in these patients were considered to be positive.
†Criteria for sensory signs: −, no sensory signs or symptoms; +, only paraesthesia or dysaesthesia; and ++, sensory deficits.
‡Deep sensory disturbance.
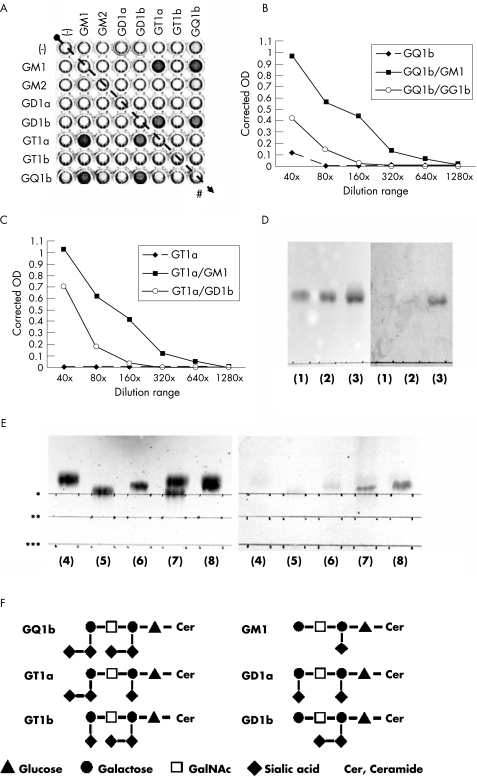
The specificity of the anti‐GSC antibodies was investigated with a representative serum sample from an anti‐GSC antibody‐positive patient with MFS (patient 10). An ELISA showed that the serum had IgG antibody activities for GQ1b/GM1, GQ1b/GD1b, GT1a/GM1 and GT1a/GD1b, but little activity against GQ1b and GT1a (fig 1A–C). Thin‐layer chromatography studies also showed specific immunoreactivity against the overlapping portion of two gangliosides, including GT1a/GM1, GQ1b/GM1 and GT1a/GD1b (fig 1D,E).
Figure 1 ELISA and thin‐layer chromatogram (TLC) immunostaining of the serum of patient 10. (A) In the ELISA, reactions in wells containing GQ1b/GM1, GQ1b/GD1b, GT1a/GM1 and GT1a/GD1b (w/w = 0.1/0.1) were much stronger than those in wells containing only GQ1b. The number of control wells (#) on the plate is indicated by oblique dotted arrows. (B,C) Serum in the ELISA was diluted serially from 1:40 to 1:1280 and the antibody titres were as follows: IgG anti‐GQ1b/GM1, 1:320; anti‐GQ1b/GD1b, 1:80, anti‐GQ1b, 1:40; anti‐GT1a/GM1, 1:320; anti‐GT1a/GD1b, 1:80; and anti‐GT1a, negative results. (D,E) In TLC immunostaining, the developing solvent consisted of chloroform, methanol and 0.2% CaCl2·2H2O (50:45:10, v/v). The following ganglioside antigens were developed in each lane: (1) GT1a (2 μg), (2) GD1b (2 μg), (3) GT1a and GD1b (2 μg each), (4) GM1 (2 μg), (5) GT1a (2 μg), (6) GQ1b (2 μg), (7) GT1a and GM1 (2 μg each) and (8) GQ1b and GM1 (2 μg each). The start line for each antigen is indicated by asterisks: (*) for GQ1b, (**) for GT1a and (***) for GM1. Serum was diluted 1:100. Peroxidase‐conjugated goat anti‐human IgG Fc antibody (ICN Biomedicals, Aurora, Ohio, USA; diluted 1:200) was used as the secondary antibody. Left panel: TLC results visualised by orcinol reagent; right panel: TLC immunostaining. Positive immunostaining in lane (3) on the right panel indicates an antibody reaction to the GT1a–GD1b complex (GT1a/GD1b) (D). Positive staining in lanes (7) and (8) on the right panel indicates antibody reactions to GT1a/GM1 and GQ1b/GM1, respectively (E). Antibody reactions to GT1a, GD1b, GM1, GT1a and GQ1b were negative. (F) Carbohydrate structures of GQ1b, GT1a, GD1b, GM1, GT1b and GD1a are shown. OD, optical density.
An immunoabsorption study with serum from patient 10 showed that the serum antibodies were specifically immunoreactive with GQ1b/GM1 and GT1a/GM1, but not with GM1 or GT1a, although the antibodies appeared to cross react with GQ1b. The percentage absorption of anti‐GQ1b/GM1 antibody activity was 1.8% by the GM1 antigen, 43% by GQ1b, 8.8% by GT1a, 96% by GQ1b/GM1 and 83% by GT1a/GM1. The percentage absorption of anti‐GT1a/GM1 antibody activity was 7.6% by GM1, 47% by GQ1b, 8.5% by GT1a, 94% by GQ1b/GM1 and 70% by GT1a/GM1.
Fine specificity of anti‐GSC antibodies and clinical features of anti‐GSC antibody‐positive patients with MFS
The ELISA showed that the fine specificity of serum antibodies in patients with MFS was heterogeneous (table 1). Some serum antibodies had stronger activity with GQ1b/GM1 or GT1a/GM1 than with either GQ1b or GT1a alone, whereas others had little or no activity against GQ1b/GM1 or GT1a/GM1, despite showing intense activity with GQ1b or GT1a. On the basis of the presence of anti‐GSC antibodies, the 12 patients with MFS could be subdivided into the three groups: anti‐GSC negative (patients 1–5), anti‐GQ1b/GM1 positive (patients 6–10) and anti‐GQ1b/GD1a positive (patients 11 and 12; table 1).
Sensory signs were infrequent in patients with MFS with antibodies to GQ1b/GM1 and GT1a/GM1 (table 1), but otherwise there were no remarkable differences in clinical features between anti‐GSC antibody‐positive and antibody‐negative patients with MFS. All the patients had antecedent respiratory infections: Haemophilus influenzae was identified from a throat swab of patient 4 and influenza B virus was serologically proved to be a pathogen in the antecedent infection of patient 7.
Discussion
This study confirmed that the anti‐GQ1b antibody is a useful marker for MFS, but the fine specificity of anti‐ganglioside antibodies in MFS was more diverse than expected. Antibodies to GSCs containing GQ1b or GT1a, and anti‐GQ1b and anti‐GT1a antibodies, may be crucial for the development of MFS. Antecedent respiratory infection in patients with MFS may be associated with production of antibodies to GSCs containing GQ1b or GT1a.
According to the results of the ELISA, serum antibodies in five patients (6–10) most strongly bound to GQ1b/GM1 and GT1a/GM1. Hence, a combination of [Galβ1–3GalNAc] and [NeuAcα2–8NeuAcα2–3Galβ1–3GalNAc] in the terminal residues of gangliotetraose structures may be important as an antigenic epitope for those anti‐GSC antibodies (fig 1F). Serum antibodies in patients 11 and 12 bound most strongly to GQ1b/GD1a and GQ1b/GT1b, and a combination of [NeuAcα2–3Galβ1–3GalNAc] and [NeuAcα2–8NeuAcα2–3Galβ1–3GalNAc] in the terminal residues may be a target antigen in those patients. On the other hand, sera from patients 1–4 had antibodies to GQ1b or GT1a but not to GSCs, and showed attenuation of these activities on addition of another ganglioside antigen, suggesting that the serum antibodies had monospecific activities to GQ1b or GT1a. Thus, there are at least three different specificities in MFS‐associated antibodies. Such differences in antibody specificity seem to have some influence on clinical features in MFS, because sensory signs were infrequent in patients with MFS with antibodies specific to GQ1b/GM1 and GT1a/GM1, but were commonly seen in patients with other antibodies. The clinical relevance of such anti‐GSC antibodies, however, needs to be investigated in a larger number of patients with MFS before firm conclusions can be drawn.
Much evidence has indicated a pathophysiological role for the IgG anti‐GQ1b antibody in the development of MFS. Immunohistochemical, ex vivo or in vitro studies with monoclonal anti‐GQ1b antibody have shown not only specific localisation of GQ1b in the human peripheral nerves but also the neuroparalytic action of the anti‐GQ1b antibodies, such as conduction block at motor nerve terminals.5,6,10,11,12,13 Similar studies with antibodies monospecific to GSCs comprising GQ1b and GT1a may be useful for elucidation of the pathogenetic mechanisms of MFS.
The characteristic formation of clusters of gangliosides in the plasma membrane may result in anti‐GSC antibodies causing nerve dysfunction more efficiently than monospecific anti‐GQ1b antibody. Whether anti‐GSC and anti‐GQ1b antibodies bind to identical sites in neuronal membranes remains unclear, and future investigations on the localisation and possible roles of GSCs in the plasma membrane are required to deal with this issue.
Acknowledgements
We thank Ms Miwako Suemura for her assistance with the ELISAs and TLC immunostaining, and Ms Masami Sada for data collection. This research was supported in part by Grants‐in‐Aid for Scientific Research (14570581 and 16590854) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a Research Grant for Neuroimmunological Diseases and a Health Sciences Research Grant (Research on Psychiatric and Neurological Diseases and Mental Health) from the Ministry of Health, Labour and Welfare of Japan.
Abbreviations
GBS - Guillain–Barré syndrome
GSC - ganglioside complex
MFS - Miller Fisher syndrome
Footnotes
Competing interests: None.
References
- 1.Kaida K, Morita D, Kanzaki M.et al Ganglioside complexes: as new target antigens in Guillain‐Barré syndrome. Ann Neurol 200456567–571. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997387569–572. [DOI] [PubMed] [Google Scholar]
- 3.Willison H J, O'Hanlon G M. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol 19991003–12. [DOI] [PubMed] [Google Scholar]
- 4.Chiba A, Kusunoki S, Shimizu T.et al Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol 199231677–679. [DOI] [PubMed] [Google Scholar]
- 5.Chiba A, Kusunoki S, Obata H.et al Serum anti‐GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain‐Barré syndrome: clinical and immunohistochemical studies. Neurology 1993431911–1917. [DOI] [PubMed] [Google Scholar]
- 6.Kusunoki S, Chiba A, Kanazawa I. Anti‐GQ1b IgG antibody is associated with ataxia as well as ophthalmoplegia. Muscle Nerve 1999221071–1074. [DOI] [PubMed] [Google Scholar]
- 7.Yuki N, Susuki K, Hirata K. Ataxic Guillain‐Barré syndrome with anti‐GQ1b antibody: relation to Miller Fisher syndrome. Neurology 2000541851–1853. [DOI] [PubMed] [Google Scholar]
- 8.Kaida K, Kusunoki S, Kamakura K.et al Guillain‐Barré syndrome with antibody to a ganglioside, N‐acetylgalactosaminyl GD1a. Brain 2000123116–124. [DOI] [PubMed] [Google Scholar]
- 9.Kaida K, Kusunoki S, Kamakura K.et al Guillain‐Barré syndrome with IgM antibody to the ganglioside GalNAc‐GD1a. J Neuroimmunol 2001113260–267. [DOI] [PubMed] [Google Scholar]
- 10.Willison H J, O'Hanlon G M, Paterson G.et al A somatically mutated human antiganglioside IgM antibody that induces experimental neuropathy in mice is encoded by the variable region heavy chain gene, V1–18. J Clin Invest 1996971155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodyear C S, O'Hanlon G M, Plomp J J.et al Monoclonal antibodies raised against Guillain‐Barré syndrome‐associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle‐nerve preparations. J Clin Invest 1999104697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hanlon G M, Plomp J J, Chakrabarti M.et al Anti‐GQ1b ganglioside antibodies mediate complement‐dependent destruction of the motor nerve terminal. Brain 2001124893–906. [DOI] [PubMed] [Google Scholar]
- 13.Halstead S K, O'Hanlon G M, Humphreys P D.et al Anti‐disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain 20041272109–2123. [DOI] [PubMed] [Google Scholar]