Abstract
Background
Although the aetiology of moyamoya disease (MMD) has not been fully clarified, genetic analysis of familial MMD (F‐MMD) has considerable potential to disclose it.
Objective
To determine the inheritance pattern and clinical characteristics of F‐MMD to enable precise genetic analyses of the disease.
Methods
15 highly aggregated Japanese families (52 patients; 38 women and 14 men) with three or more affected members were examined. The difference in categories of age at onset (child onset, adult onset and asymptomatic) between paternal and maternal transmission was compared by χ2 statistics.
Results
In all families there had been three or more generations without consanguinity, and all types of transmission, including father‐to‐son, were observed. Among a total of 135 offspring of affected people, 59 (43.7%) were patients with MMD or obligatory carriers. Affected mothers were more likely to produce late‐onset (adult‐onset or asymptomatic) female offspring (p = 0.007).
Conclusions
The mode of inheritance of F‐MMD is autosomal dominant with incomplete penetrance. Thus, in future genetic studies on F‐MMD, parametric linkage analyses using large families with an autosomal dominant mode of inheritance are recommended. Genomic imprinting may be associated with the disease.
Moyamoya disease (MMD) is an idiopathic progressive angiopathy characterised by progressive stenosis or occlusion and affecting the terminal portions of the bilateral internal carotid artery and the circle of Willis. Collateral vessels develop at the base of the brain to compensate for the progressive stenosis. These enlarged collaterals appear as a puff of smoke on angiography, which gives the disease its name.
MMD is predominantly found in East Asian populations, with most reported cases originating from Japan, Korea and China. The estimated annual incidence in Japan in 1994 was 0.35 per 100 000 population,1 whereas that in Europe was one tenth of this.2 It is well known that MMD has been observed predominantly in women, with a female‐to‐male ratio of 1.8.1,3 Recently, MMD has been diagnosed by magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA),4,5 which makes it possible to detect asymptomatic patients. Accordingly, familial occurrence has been increasing; it was observed in 12.1% of the patients in 2004.3 The female predominance, East Asian distribution and familial occurrence of the disease imply the existence of genetic risk factors.
To clarify the genetic background of MMD, several non‐parametric linkage analyses using mainly affected sibling pairs have been carried out, showing linkages to 3p24.2–p26, 6q25, 8q23, 12p12 and 17q25.6,7,8,9 The association analysis of tissue inhibitor of metalloproteinase 2 in 17q25 showed that a polymorphism in the promoter region was markedly associated with familial MMD (F‐MMD).10
To make further progress in genetic analysis of the disease, it is important to enrol highly aggregated families with MMD to characterise the clinical features and inheritance patterns in F‐MMD. Some unresolved issues are as follows:
The mode of inheritance has not yet been determined.
Some reports mention that paternal transmission led to earlier onset than maternal transmission.11 However, the difference between maternal and paternal transmission has not been proved by a statistical analysis.
The aim of this study is to resolve these issues, to enable precise genetic analyses of F‐MMD.
Methods
Study population
This study was approved by the ethics committee of the Kyoto University Institutional Review Board (Kyoto, Japan), and written informed consent was obtained from all the participants. We collected cases of F‐MMD by collaborating with hospitals. These patients were diagnosed according to the official diagnostic criteria of the Research Committee on the Spontaneous Occlusion of the Circle of Willis of the Ministry of Health and Welfare, Japan.4 A clinical interview and 1.5‐T MRI and MRA examination were carried out for all available relatives. We ascertained the medical history, age, age at onset, age at diagnosis, first sign at onset, course of the disease, treatment and associated diseases. For a few twin cases of MMD, we reviewed the previous case reports. We searched PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db = PubMed) from 1968 to 2005 and the Japan Medical Abstract Society (Japana Centra Revuo Medicina, http://login.jamas.or.jp/enter.html) from 1983 to 2005 (full data) using the keyword “moyamoya twin” (“moyamoya souseiji” in Japanese). From both searches, only Japanese families were included in this study.
Data analysis
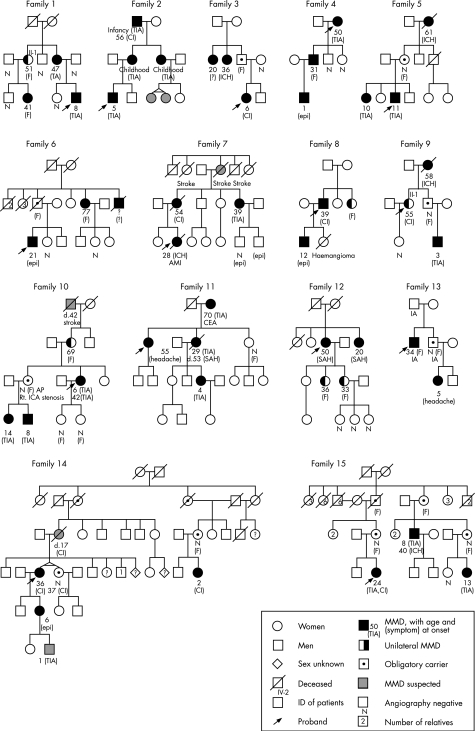
We assumed the nearest common ancestor of the affected people to be the founder of the family. We put the founder in the first generation in the pedigree chart (fig 1). So, family 1 and family 2 were treated as three‐generation families, and family 14 was treated as a five‐generation family. People who were suspected to be affected according to their symptoms but whose diagnosis had not been confirmed by MRI and MRA or conventional angiography were treated as unaffected. Patients with MMD and obligatory carriers were treated as affected. Age at onset was divided into three groups: childhood onset (<15 years old), adult onset (⩾15 years old) and adult asymptomatic patients (including obligatory carriers). The difference in age at onset between the four types of transmission (mother‐to‐daughter, mother‐to‐son, father‐to‐daughter and father‐to‐son) was analysed statistically using the χ2 test or Fisher's exact test. All statistical analyses were carried out using SAS software V.8.2.
Figure 1 Families with familial moyamoya disease (MMD). AMI, acute myocardial infarction; CEA, carotid endoarterectomy; CI, cerebral infarction; epi, epileptic attack; (F), symptom free; IA, intracranial aneurysm; ICH, intracerebral haemorrhage; Rt. ICA, right internal carotid artery; SAH, subarachnoid haemorrhage; TIA, transient ischaemic attack. Filled symbols indicate patients with MMD; half‐filled symbols, patients with unilateral MMD; dotted symbols, obligatory carriers; gray‐shaded symbols, people suspected to be affected with MMD; circles, women; squares, men; diamonds, people of unknown sexuality; crossed symbols, deceased people; arrows, probands; numbers in the right upper angle of symbols, ID of patients; numbers in symbols, number of relatives; N, people who were confirmed to be unaffected by angiography. Numbers below the circle show age at onset, and symptom at the onset was shown in a parenthesis.
Results
We studied 15 families (fig 1), including five families previously reported elsewhere.12,13,14,15,16 Twelve families were three‐generation families, two were four‐generation and one was a five‐generation family. No families showed parental consanguinity. We studied a total of 52 patients (38 women and 14 men; sex ratio 2.71) and 14 obligatory carriers (9 women and 5 men). Among 14 obligatory carriers, 8 were diagnosed as not having MMD by MRI and MRA or angiography, and 6 did not receive diagnostic examinations (some may have been asymptomatic patients). The number of children of affected people was 135 (84 women and 51 men; sex ratio 1.65), among whom 59 (43.7%) were affected. All types of transmission, including father‐to‐son, were observed (table 1). The ratio of maternal transmission to paternal transmission was 3.44, showing maternal predominance, and mother‐to‐daughter transmission was most commonly seen (60.0%). No difference in the category of age at onset was observed between paternal and maternal transmission, although a significant difference was observed between mother‐to‐daughter transmission and the other patterns of transmission (table 1; p = 0.028). Adult onset and asymptomatic patients were more commonly seen with mother‐to‐daughter transmission than with the other types of transmission (p = 0.007) (see fig 1).
Table 1 Differences in age at onset with four types of transmission.
| F−F | F−M | M−F | M−M | Maternal | Paternal | p Value | F−F | Non‐F−F | p Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total number | 25 | 7 | 6 | 4 | 32 | 10 | 25 | 17 | ||
| Class of age at onset | ||||||||||
| Child onset (0–14 years) | 7 | 5 | 4 | 3 | 12 | 7 | 0.193* | 7 | 12 | 0.028* |
| Adult onset (⩾15 years) | 10 | 1 | 0 | 1 | 11 | 1 | 10 | 2 | ||
| Asymptomatic† | 8 | 1 | 2 | 0 | 9 | 2 | 8 | 3 | ||
| Child onset | 12 | 7 | 0.108 | 7 | 12 | 0.011 | ||||
| Adult onset | 11 | 1 | 10 | 2 | ||||||
| Child onset | 12 | 7 | 0.144 | 7 | 12 | 0.007 | ||||
| Non‐child onset | 20 | 3 | 18 | 5 |
F−F, female‐to‐female transmission; F−M, female‐to‐male transmission; M−F, male‐to‐female transmission; M−M, male‐to‐male transmission. Non‐F−F consists of F−M, M−F and M−M.
*Calculated comparing three classes of age at onset; †includes asymptomatic patients with MMD and obligatory carriers. Non‐child onset indicates adult onset, asymptomatic and obligatory carriers. p Values were calculated by χ2 statistics or Fisher's exact test.
Familial occurrence of both unilateral and bilateral MMD was observed in five families. There were six patients with unilateral MMD, and all of them showed adult onset. In family 1, patient II‐1 was diagnosed with probable MMD at 51 years of age and remained unilateral for >18 years. In family 9, patient II‐1 remained unilateral for >4 years. No follow‐up data were available for the other patients (see fig 1).
In the literature, we found 17 twin pairs, including ours.17,18,19,20,21,22,23,24,25,26,27,28,29,30 Of 17 pairs, 14 were monozygotic, two were dizygotic and one was of unknown zygosity. The ratio of monozygotic to dizygotic twinning was about 2:1 in the general population,31 whereas that in patients with MMD was 4.7–7.5:1, showing monozygotic predominance. All 12 monozygotic twins were female except for two pairs of unknown sexuality. Among the 12 monozygotic twins, 5 pairs showed discordant phenotypes (symptomatic v asymptomatic, bilateral v unilateral, or patients v non‐patients). In family 14, for example, one of the twins was a patient whereas the other was not.
Discussion
Mode of inheritance
Owing to the lack of highly aggregated families with MMD, its mode of inheritance has remained undetermined. We studied as many as 15 large families with three or more generations. Parental consanguinity was absent from these families, which excluded the possibility of autosomal recessive inheritance. The families exhibited all types of transmission, including father‐to‐son, which made X‐linked inheritance unlikely. As for the proportion of affected people among the total offspring, 50% indicates an autosomal dominant mode and 25% indicates an autosomal recessive mode of inheritance. In our patients, nearly one half of the children of affected people have become affected, which supports an autosomal dominant model with incomplete penetrance. Although the pedigree analysis carried out by Seol et al32 yielded no specific pattern of inheritance in F‐MMD, they recruited only small families with two affected members in each family, which made it difficult to tell the pattern of inheritance.
Genomic imprinting and epigenetic modification
We have shown that transmission is predominantly maternal and that affected mothers were more likely to produce late‐onset (adult‐onset or asymptomatic) female offspring. This non‐mendelian sex‐related pattern of inheritance is referred to as “parent‐of‐origin effect”. One explanation for the effect is genomic imprinting, the molecular basis of which is epigenetic mechanisms such as DNA methylation and histone acetylation.33 It is noteworthy that the Prader−Willi syndrome and Angelman's syndrome, which are well known for their genomic imprinting, are reported to be associated with moyamoya angiopathy.34
It is well known that patients with MMD are predominantly female. We have shown that the children of affected people are also predominantly female, whether affected or not, and that most monozygotic twins were female. This suggests that a gene responsible for MMD may be associated with sex determination.
We have shown that the proportion of monozygotic twinning is higher in MMD than in the general population. Most monozygotic twin pairs were female, and there were several pairs of monozygotic twins who were discordant for MMD. A good example of female‐dominant monozygotic twins with discordant phenotypes is the Beckwith−Wiedemann syndrome, which is also characterised by genomic imprinting. Weksberg et al35 reported that a loss of imprinting in the pre‐implantation period would be a credible aetiology for the twinning as well as the discordances between monozygotic twins. Another example is Noonan's syndrome, which is caused by a mis‐sense mutation in PTPN11.36 The PTPN11 gene has been implicated in oogenesis in Caenorhabditis elegans, and is thought to be associated with oogenesis and twinning in humans.36 Noonan's syndrome is known to cause moyamoya angiopathy,37 indicating that MMD might be associated with the genes that control twinning.
Thus, in addition to genetic risk factors, epigenetic factors such as genomic imprinting, sex determination and twinning may be associated with the disease.
Unilateral MMD
Although MMD is defined as a bilateral lesion, unilateral involvement also occurs. Most paediatric patients with unilateral MMD develop a bilateral lesion within 1–2 years, whereas lesions in adults tend to remain unilateral.38 The official diagnostic criteria of the Research Committee on the Spontaneous Occlusion of the Circle of Willis of the Ministry of Health and Welfare classify adult cases with bilateral occlusive lesions as “definite” MMD and those with unilateral involvement as “probable” MMD. We investigated six cases of familial occurrence of probable MMD. The coincidence of probable and definite MMD in a single family indicates that they reflect different phenotypes caused by the same genetic defects.
Future linkage analysis
Although previous studies shared several pedigrees in their non‐parametric linkage analyses, they showed different linkage regions. There seem to be three main explanations for this phenomenon. Firstly, MMD may be caused by several different mechanisms (disease heterogeneity). Secondly, MMD exhibits different modes of inheritance—namely, autosomal dominant and autosomal recessive (genetic heterogeneity). Thirdly, several genetic factors in different loci can cause the same disease (locus heterogeneity).
In addition to the issue of heterogeneity, we have shown that the disease may be susceptible to epigenetic modifications. Therefore, the only feasible approach to identify the gene responsible would be positional cloning. To solve the issue of genetic heterogeneity, we need to collect large families of three or more generations without consanguinity, in which the autosomal dominant mode can be safely assumed. Linkage analysis of large families gives us a relatively high logarithm of odds score in each family, which makes clear the existence of disease heterogeneity or locus heterogeneity. Given the reduced penetrance, affected member‐only analysis may be the most rational approach, in which obligatory carriers should be treated as “affected”. Patients with unilateral MMD should also be included in the analysis.
In the genetic study on intracranial aneurysm, we applied a similar approach by collecting 29 highly aggregated Japanese families. We were able to successfully show the linkage to chromosome 17 and identified TNFRSF13B as a candidate gene.39,40,41
Conclusions
Pedigree analysis of highly aggregated Japanese families with MMD indicates that the mode of inheritance is autosomal dominant with reduced penetrance. Thus in future genetic studies on F‐MMD, parametric linkage analyses using large families in which the autosomal dominant mode is safely assumed are recommended. Epigenetic modification such as genomic imprinting may be associated with the disease.
Acknowledgements
This work was supported by a grant from the Ministry of Education, Science, Sports and Culture of Japan (Kiban Kenkyuu S: 17109007) and a grant from the Japan Society for the Promotion of Science (17019034), and partly supported by the Research Committee on Moyamoya Disease of the Ministry of Health, Labor and Welfare, Japan. YM is a member of JSPS research fellows. We thank the following doctors for patient recruitment and help with the MRA examinations: Akira Handa and Hiroyasu Akutsu (Okayama Rosai Hospital, Okayama, Japan), Hajime Touho (Touho Neurosurgery Clinic, Osaka, Japan), Hidenori Miyake and Yasushi Ueno (Hamamatsu Rosai Hospital, Hamamatsu, Japan), Hidekazu Nogaki (Wadayama Hospital, Wadayama, Japan), Junji Kitamura (Kitamura Clinic, Kobe, Japan), Kiyohiro Houkin (Sapporo Medical University Hospital, Sapporo, Japan), Makoto Sonobe (Mito Medical Center, Higashi‐ibaraki, Japan), Mitsuru Kimura, Hajime Iguchi and Kazuki Yamamoto (Nishiwaki Municipal Hospital, Nishiwaki, Japan), Nobuhisa Mabuchi (Soseikai General Hospital, Kyoto, Japan), Nobuyuki Sakai (Kobe City General Hospital, Kobe, Japan), Sadahiko Ban (Kobe City Public Health Center, Kobe, Japan), Satoshi Kuroda (Hokkaido University Graduate School of Medicine, Sapporo, Japan), Shigetoshi Takaya (Tango Central Hospital, Kyoto, Japan), Tatsuhito Yamagami (Kizugawa Hospital, Kyoto, Japan), Yoshihiro Ohyama (Akishima Sougo Hospital, Akishima, Japan), Yoshihiko Kamimura (Sakaide Kaisei Hospital, Sakaide, Japan), Yukio Wakuta (Saiseikai Yamaguchi General Hospital, Yamaguchi, Japan), Shigeki Yamada (Shiga Medical Center for Adults, Moriyama, Japan) and Keisuke Yamada (Kyoto University Hospital, Kyoto, Japan).
Abbreviations
F‐MMD - familial moyamoya disease
MMD - moyamoya disease
MRA - magnetic resonance angiography
MRI - magnetic resonance imaging
Footnotes
Competing interests: None declared.
References
- 1.Wakai K, Tamakoshi A, Ikezaki K.et al Epidemiological features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg 199799(Suppl 2)P1–P5. [DOI] [PubMed] [Google Scholar]
- 2.Yonekawa Y, Ogata N, Kaku Y.et al Moyamoya disease in Europe, past and present status. Clin Neurol Neurosurg 199799(Suppl 2)P58–P60. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji I, Kuriyama S, Kusaka Y.et al Epidemiological and clinical analyses of moyamoya disease in 2004. Annual report 2004 of the Research Committee on Moyamoya Disease (Spontaneous Occlusion of the Circle of Willis) of Health and Labour Sciences Research Grants, Research on measures for intractable diseases 200513–17.
- 4.Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya' disease): Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin Neurol Neurosurg 199799(Suppl 2)P238–P240. [PubMed] [Google Scholar]
- 5.Houkin K. Novel MRA staging of moyamoya disease. Annual report 2001 of the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of Ministry of Health Labour and Welfare 2002:39–43 (In Japanese with English abstract)
- 6.Ikeda H, Sasaki T, Yoshimoto T.et al Mapping of a familial moyamoya disease gene to chromosome 3p24.2–p26. Am J Hum Genet 199964533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue T K, Ikezaki K, Sasazuki T.et al Linkage analysis of moyamoya disease on chromosome 6. J Child Neurol 200015179–182. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Tada M, Houkin K.et al Linkage of familial moyamoya disease (spontaneous occlusion of the circle of Willis) to chromosome 17q25. Stroke 200031930–935. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai K, Horiuchi Y, Ikeda H.et al A novel susceptibility locus for moyamoya disease on chromosome 8q23. J Hum Genet 200449278–281. [DOI] [PubMed] [Google Scholar]
- 10.Kang H S, Kim S K, Cho B K.et al Single nucleotide polymorphisms of tissue inhibitor of metalloproteinase genes in familial moyamoya disease. Neurosurgery 2006581074–1080. [DOI] [PubMed] [Google Scholar]
- 11.Nanba R, Kuroda S, Ishikawa T.et al Familial moyamoya disease—clinical features and current study. (In Japanese) No Shinkei Geka 2004327–16. [PubMed] [Google Scholar]
- 12.Akutsu H, Sonobe M, Sugita K.et al Familial association of basilar bifurcation aneurysm and moyamoya disease—four case reports. Neurol Med Chir (Tokyo) 200343435–438. [DOI] [PubMed] [Google Scholar]
- 13.Ban S, Yamamot T, Nakao T.et al A familial case of moyamoya disease; a mother, her daughter and second cousin. (In Japanese) Clin Neurol 198525503 [Google Scholar]
- 14.Kusaka N, Tamiya T, Adachi Y.et al Adult unilateral moyamoya disease with familial occurrence in two definite cases: a case report and review of the literature. Neurosurg Rev 20062982–87. [DOI] [PubMed] [Google Scholar]
- 15.Nakaho T, Nogaki H, Saitou M.et al Three cases of moyamoya disease found in a single family. Kouritsu Toyooka Byoin Kiyo 1989155–60. [Google Scholar]
- 16.Shose Y, Kimura M, Ikeda K.et al Familial occurence of the spontaneous occlusion of the circle of Willis. Nishiwakishiritsu Nishiwaki Byoinshi 2001119–22. [Google Scholar]
- 17.Yamada H, Nakamura S, Kageyama N. Moyamoya disease in monovular twins: case report. J Neurosurg 198053109–112. [DOI] [PubMed] [Google Scholar]
- 18.Sonobe M, Takahashi S, Urakawa Y.et al “Moyamoya” disease found in identical twins. (In Japanese with English abstract) No Shinkei Geka 198081183–1188. [PubMed] [Google Scholar]
- 19.Kashihara M, Oki H, Sasaki K.et al Adult identical twins with moyamoya disease. (In Japanese with English abstract) No Shinkei Geka 1984121425–1431. [PubMed] [Google Scholar]
- 20.Kawano H, Kobayashi H, Kabuto M.et al “Moyamoya” disease in identical twins—case report. (In Japanese with English abstract) Shoni No Noshinkei 198410307–313. [Google Scholar]
- 21.Ohsawa M, Tada T, Momose G.et al Moyamoya disease in identical twins. (In Japanese) Shinsyu Igaku Zasshi 198634392 [Google Scholar]
- 22.Inugami A, Uemura K, Ogawa T.et al “Moyamoya” disease in twins. (In Japanese) Rinsho Hoshasen 198732190–192. [Google Scholar]
- 23.Kawamura S, Hadeishi H, Suzuki A.et al Moyamoya disease in twins. (In Japanese with English abstract) No To Shinkei 198739119–125. [PubMed] [Google Scholar]
- 24.Handa H, Nishikawa K, Munaka M.et al “Moyamoya” disease in identical twins—a case report. Annual report 1988 of the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of Ministry of Health Labour and Welfare (In Japanese with English abstract) 198924–26.
- 25.Houkin K, Tanaka N, Takahashi A.et al Familial occurrence of moyamoya disease. Magnetic resonance angiography as a screening test for high‐risk subjects. Childs Nerv Syst 199410421–425. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu M, Hamano S, Nara T.et al “Moyamoya disease” in identical twins in their infancy. (In Japanese) Shoni Naika 199426120–123. [Google Scholar]
- 27.Suzuki S, Morioka T, Matsushima T.et al Moyamoya disease associated with persistent primitive trigeminal artery variant in identical twins. Surg Neurol 199645236–240. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda E. Moyamoya disease in siblings. (In Japanese) Clin Neurol 197717420 [Google Scholar]
- 29.Asami N, Miyahara S, Ueda T.et al Moyamoya disease in fraternal twins. (In Japanese with English abstract) No To Shinkei 1990421093–1096. [PubMed] [Google Scholar]
- 30.Nagao K, Takahashi I, Kikuchi M.et al Moyamoya disease in identical twins and their elder sister. (In Japanese) Shonika 19932939 [Google Scholar]
- 31.Imaizumi Y, Nonaka K. The twinning rates by zygosity in Japan, 1975–1994. Acta Genet Med Gemellol (Roma) 1997469–22. [DOI] [PubMed] [Google Scholar]
- 32.Seol H J, Wang K C, Kim S K.et al Familial occurrence of moyamoya disease: a clinical study. Childs Nerv Syst. Published Online First: 25 March 2006. doi: 10.1007/s00381‐006‐0089‐4 [DOI] [PubMed]
- 33.Wolffe A P, Matzke M A. Epigenetics: regulation through repression. Science 1999286481–486. [DOI] [PubMed] [Google Scholar]
- 34.Kusuhara T, Ayabe M, Hino H.et al A case of Prader‐Willi syndrome with bilateral middle cerebral artery occlusion and moyamoya phenomenon. (In Japanese) Rinsho Shinkeigaku 199636770–773. [PubMed] [Google Scholar]
- 35.Weksberg R, Shuman C, Caluseriu O.et al Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith‐Wiedemann syndrome. Hum Mol Genet 2002111317–1325. [DOI] [PubMed] [Google Scholar]
- 36.Schollen E, Matthijs G, Gewillig M.et al PTPN11 mutation in a large family with Noonan syndrome and dizygous twinning. Eur J Hum Genet 20031185–88. [DOI] [PubMed] [Google Scholar]
- 37.Ganesan V, Kirkham F J. Noonan syndrome and moyamoya. Pediatr Neurol 199716256–258. [DOI] [PubMed] [Google Scholar]
- 38.Kawano T, Fukui M, Hashimoto N.et al Follow‐up study of patients with “unilateral” moyamoya disease. Neurol Med Chir (Tokyo) 199434744–747. [DOI] [PubMed] [Google Scholar]
- 39.Yamada S, Utsunomiya M, Inoue K.et al Genome‐wide scan for Japanese familial intracranial aneurysms: linkage to several chromosomal regions. Circulation 20041103727–3733. [DOI] [PubMed] [Google Scholar]
- 40.Mineharu Y, Inoue K, Inoue S.et al Association analysis of common variants of ELN, NOS2A, APOE, and ACE2 to intracranial aneurysm. Stroke 2006371189–1194. [DOI] [PubMed] [Google Scholar]
- 41.Inoue K, Mineharu Y, Inoue S.et al Search on chromosome 17 centromere reveals TNFRSF13B as a susceptibility gene for intracranial aneurysm: a preliminary study. Circulation 20061132002–2010. [DOI] [PubMed] [Google Scholar]