Abstract
Background
Normal pressure hydrocephalus (NPH) is associated with corpus callosum abnormalities.
Objectives
To study the clinical and neuropsychological effect of callosal thinning in 18 patients with idiopathic NPH and to investigate the postsurgical callosal changes in 14 patients.
Methods
Global corpus callosum size and seven callosal subdivisions were measured. Neuropsychological assessment included an extensive battery assessing memory, psychomotor speed, visuospatial and frontal lobe functioning.
Results
After surgery, patients showed improvements in memory, visuospatial and frontal lobe functions, and psychomotor speed. Two frontal corpus callosum areas, the genu and the rostral body, were the regions most related to the clinical and neuropsychological dysfunction. After surgery, total corpus callosum and four of the seven subdivisions presented a significant increase in size, which was related to poorer neuropsychological and clinical outcome.
Conclusion
The postsurgical corpus callosum increase might be the result of decompression, re‐expansion and increase of interstitial fluid, although it may also be caused by differences in shape due to cerebral reorganisation.
Ventricular dilatation and corpus callosum abnormalities are the anatomical changes most often reported in association with normal pressure hydrocephalus (NPH). Callosal abnormalities include changes in the morphology and the magnetic resonance imaging (MRI) signal. The most consistent findings are stretching, uniform and focal thinning, and upward elevation.1,2,3,4 Callosal damage has been primarily attributed to lateral ventricle dilatation1 and to the impingement of the corpus callosum against the falx.2
Two studies indicated a partial or complete recovery of different callosal parameters after shunt surgery.4,5 However, in these studies, only five and eight patients with different types of hydrocephalus were analysed postsurgically. Other studies have reported corpus callosum abnormalities after surgery in 3–17% of cases, including signal changes,6,7,8,9,10 a transient scalloping deformity6 and increased thickness.7,8 Although the nature of the postsurgical changes in the corpus callosum remains unclear, several causes have been suggested, including callosal compression against the falx, decompression and overdrainage.6,7,8,9
To our knowledge, no morphological MRI quantitative studies of corpus callosum in NPH have been performed to date, nor has the possible involvement of the corpus callosum in the neuropsychological deterioration in NPH been investigated in depth. As damage to the corpus callosum can affect cognition,11 the aim of this study was to investigate the contribution of corpus callosum thinning to the neuropsychological deficits in NPH and to determine the postsurgical callosal changes and their relationship with cognitive outcome.
Methods
Patients
Eighteen consecutive patients (nine men and nine women) with idiopathic NPH, shunted between March 2001 and October 2002, were included in this study. All patients had ventricular dilatation (Evans' index ⩾0.30) and presented the clinical triad, except three patients who had no sphincter disturbances. Eleven patients were dependent on others for daily life activities (Stein and Langfit's Scale grade IV) and seven required some help or supervision (grades II and III). Mean evolution time was 29 (standard deviation (SD) 15.97; range 12–60) months. Mean age was 74.56 (SD 7.06; range 59–83) years and mean years of education was 7.83 (SD 6.13; range 1–30).
In accordance with our criteria for shunting,12 all patients had >10% of B‐waves in the continuous intracranial pressure recording with or without abnormal resistance to outflow of cerebrospinal fluid (>10 mm Hg/ml/min on Katzman's infusion test). The mean resistance to outflow was 17.5 (SD 4.8; range 7.5–25.6) mm Hg/ml/min and the mean percentage of B‐waves was 48% (SD 19.9%; range 18–98%). In all 18 patients, a differential low‐pressure valve system was implanted with an in‐line or incorporated gravity‐compensating device. Informed consent for all aspects of the study was obtained from each patient or a close relative.
There was no treatment‐related mortality. A small and asymptomatic subdural collection (self‐limiting hygroma) was diagnosed in one patient 6 months after shunting. Three patients could not attend the neuroimaging and neuropsychological follow‐up (one died, two patients in a residential setting), and another patient did not attend the MRI control.
NEUROPSYCHOLOGICAL ASSESSMENT, CLINICAL EVALUATION, AND EVALUATION OF DAILY LIFE ACTIVITIES
Patients were administered a neuropsychological test battery and several clinical and functional scales sensitive to NPH dysfunction presurgically and 6 months after surgery by the same examiner (MM) who was blinded to the neuroimaging results (table 1).12,13
Table 1 Neuropsychological and neuroimaging results.
| n | Presurgery | Postsurgery | p value | 95% CI for the difference | Effect size | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Mean (SD) | Median | |||||
| Attention/memory | ||||||||
| Mental control | 15 | 3 (1.77) | 4 | 3.6 (1.5) | 4 | NS | –1.76 to 0.56 | |
| Digit span forward | 15 | 4.27 (0.59) | 4 | 4.47 (0.64) | 4 | NS | –0.57 to 0.17 | |
| AVLT total | 15 | 19.93 (7.62) | 21 | 23.47 (10.01) | 23 | NS | –8.16 to 1.1 | |
| AVLT long‐term | 15 | 2 (2.14) | 2 | 3.73 (3.49) | 3 | 0.004* | –2.81 to –0.66 | 0.68 |
| Visual Retention I | 15 | 12.93 (6.34) | 14 | 20.27 (6.11) | 21 | 0.002* | –11.59 to –3.08 | 0.72 |
| Visual Retention II | 15 | 5 (7.04) | 0 | 9.53 (7.05) | 10 | 0.027† | –8.48 to –0.59 | 0.55 |
| Visuospatial | ||||||||
| Line orientation | 15 | 10.6 (8.07) | 6 | 17.47 (7.88) | 19 | 0.012* | –11.98 to –1.75 | 0.61 |
| Block design | 15 | 6.53 (2.59) | 7 | 8.93 (2.99) | 10 | 0.004* | –3.91 to –0.89 | 0.67 |
| Frontal | ||||||||
| Fluency (FAS) | 15 | 10 (6.89) | 9 | 14.27 (8.8) | 15 | 0.018† | –7.68 to –0.85 | 0.58 |
| Fluency (animals) | 15 | 9.73 (2.71) | 10 | 10.67 (5.09) | 13 | NS | –3 to 1.13 | |
| Trail‐making Test B | 10 | 402.9 (54.08) | 420 | 346.90 (100.68) | 408.5 | NS | –8.33 to 120.33 | |
| Digit span backward | 15 | 2.33 (0.49) | 2 | 2.93 (0.46) | 3 | ⩽0.001 | –0.88 to –0.32 | 0.77 |
| Stroop Test | 12 | 4133.15 (3971.66) | 2963.65 | 3056.51 (1262.49) | 2825.86 | NS | –1600.94 to 3754.24 | |
| Psychomotor speed | ||||||||
| Trail‐making Test A | 14 | 232.57 (98.45) | 207.5 | 164.86 (101.27) | 150.5 | 0.011* | 18.24 to117.19 | 0.63 |
| Pegboard right | 14 | 7.5 (2.85) | 7.5 | 9.21 (2.46) | 10 | 0.004* | –2.76 to –0.67 | 0.7 |
| Pegboard left | 13 | 6.23 (3.37) | 5 | 8.62 (2.47) | 9 | 0.015† | –4.22 to –0.55 | 0.63 |
| General | ||||||||
| MMSE | 15 | 23.07 (4.65) | 26 | 25.47 (3.42) | 26 | 0.048† | –4.78 to –0.02 | 0.5 |
| Clinical | ||||||||
| NPH | 17 | 8.82 (2.38) | 8 | 12.76 (2.14) | 13 | ⩽0.001 | –5.34 to –2.54 | 0.83 |
| NPH gait‡ | 17 | 2.94 (0.9) | 3 (IQR 1) | 4.12 (0.7) | 4 (IQR 1) | ⩽0.001 | ||
| NPH cognition‡ | 17 | 3.29 (0.85) | 4 (IQR 2) | 4.29 (0.85) | 4 (IQR 1) | 0.003* | ||
| NPH sphincter‡ | 17 | 2.59 (1.37) | 2 (IQR 2) | 4.35 (1.06) | 5 (IQR 1) | ⩽0.001 | ||
| Stein‡ | 15 | 3.27 (0.88) | 4 (IQR 2) | 1.53 (1.25) | 1 (IQR 1) | 0.003* | ||
| Daily life activities‡ | 15 | 2.73 (3.15) | 1 (IQR 7) | 7.47 (3.07) | 9 (IQR 4) | 0.002* | ||
| CC | ||||||||
| Rostrum | 14 | 37.05 (15.15) | 32.25 | 31.87 (14.67) | 30.93 | NS | –3.34 to 10.69 | |
| Genu | 14 | 75.74 (21.33) | 78.2 | 91.52 (31.91) | 87.48 | 0.008* | –26.68 to –4.88 | 0.66 |
| Rostral body | 14 | 74.73 (19.58) | 79.97 | 76.62 (17.98) | 80.85 | NS | –12.18 to 8.39 | |
| Anterior midbody | 14 | 51.88 (11.53) | 50.37 | 56.87 (13.4) | 57.44 | NS | –10.4 to 0.43 | 0.67 |
| Posterior midbody | 14 | 48.04 (11.14) | 51.25 | 57.31 (15.5) | 57.88 | 0.007* | –15.47 to –3.06 | 0.72 |
| Isthmus | 14 | 46.83 (10.73) | 47.72 | 60.59 (18.84) | 55.67 | 0.002* | –21.63 to –5.89 | 0.66 |
| Splenium | 14 | 131.72 (24.28) | 131.66 | 144.53 (25.87) | 148.89 | 0.008* | –21.67 to –3.96 | 0.71 |
| Total CC | 14 | 465.99 (83.65) | 479.79 | 519.3 (84.27) | 507.18 | 0.003* | –85.1 to –21.53 | –85.1 |
AVLT, Auditory–Verbal Learning Test; CC, corpus callosum; IQR, interquartile range; MMSE, Mini‐Mental State Examination; NPH, normal pressure hydrocephalus; RDRS, Rapid Disability Rating Scale.
*p⩽0.01.
†p⩽0.05.
‡Non‐parametric Wilcoxon matched‐pairs signed ranks test.
Image acquisition and morphological measure
Three‐dimensional T1‐weighted MRI were acquired on a 1.5‐T Signa General Electric (Milwaukee, Wisconsin, USA) using a three‐dimensional fast spoiled gradient recall sequence, with the following parameters: TR = 16.8 ms, TE = 5.1 ms, TI = 300 ms, field of view = 24×24, slice thickness = 1.5 mm, 0‐gap neuronal helix–loop–helix protein = 1, matrix = 256×192. In total, 100–128 contiguous slices were obtained.
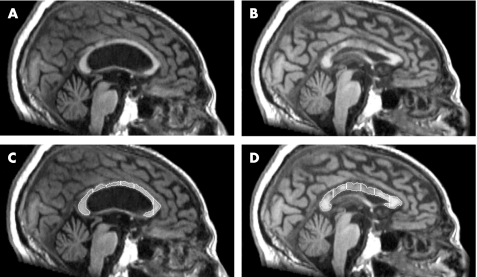
Magnetic resonance measures were obtained with the Analyze V.5.0 software (Biomedical Imaging Resource, Mayo Foundation, Rochester, Minnesota, USA). A mid‐sagittal slice was selected to outline the corpus callosum, which was semiautomatically segmented in seven parts (rostrum, genu, rostral body, anterior midbody, posterior midbody, isthmus and splenium) following the approach described by Witelson14 (fig 1). Values obtained were divided by the total intracranial area to ensure comparability between patients. Total intracranial area was estimated from the same mid‐sagittal slice used for the quantification of the corpus callosum area. All measures were made by the same investigator (RP), who was blinded to the patients' characteristics.
Figure 1 Presurgical (A) and postsurgical incremented (B) corpus callosum images. (C,D) Corpus callosum of the same patient showing the seven anatomical subdivisions measured.
Statistical analysis
To compare preoperative and postoperative data, we used the paired Student's t–test, and showed the confidence interval (CI) for the difference and the effect size in each case. For the ordinal variables, the non‐parametric Wilcoxon matched‐pairs signed ranks test was used. For correlational analysis, we used Pearson's correlation coefficient for the interval variables and Spearman's correlation for the ordinal variables.
We calculated the percentage of change between basal and postoperative conditions:
((postoperative – preoperative)/preoperative)×100.
Significance was set at p = 0.01.
Results
Six months after surgery, only one patient showed no improvement on the NPH scale. The remaining patients (94%) showed some clinical recovery. In all, 13 (77%) patients presented gait amelioration, 11 (65%) cognitive improvement, and 13 (93%) patients with sphincter dysfunction also improved. Patients showed improvements on some tests of memory, visuospatial and frontal lobe functions, and psychomotor speed, and on all clinical and daily life activity scales. Moreover, total corpus callosum size and four of its seven subdivisions showed a statistically significant increase from preoperational to postoperational analysis (table 1).
Before surgery, the size of different regions of the corpus callosum correlated with several measures of neuropsychological, clinical and daily life activities. Smaller corpus callosum size was related to worse functioning. The genu of the corpus callosum was related to psychomotor speed (Pegboard right hand: r = 0.64, p = 0.005, r2 = 0.41; Pegboard left hand: r = 0.59; p = 0.013, r2 = 0.35) and to clinical and daily life activity functioning (NPH cognition: rs = 0.64, p = 0.005; Stein and Langfit Scale: rs = –0.69, p = 0.002). The rostral body correlated with frontal functions (Stroop test: r = –0.65, p = 0.012, r2 = 0.42). Finally, the splenium was also related to a frontal functioning test (Trail‐making Test B: r = –0.66, p = 0.013; r2 = 0.44).
After surgery, increases in regional corpus callosum size were significantly related to lesser improvement in several neuropsychological and clinical tests. The percentage changes in the genu were related to changes in frontal functioning (Stroop Test: r = 0.85, p = 0.004, r2 = 0.72). Changes in the posterior midbody correlated with changes in memory (Visual Retention I: r = –0.69; p = 0.009, r2 = 0.49), frontal functioning (Stroop Test: r = 0.82, p = 0.006, r2 = 0.67) and clinical measures (NPH: r = –0.68, p = 0.008, r2 = 0.46). Finally, changes in the isthmus were related to changes in frontal functioning (Stroop Test: r = 0.86, p = 0.003, r2 = 0.74) and clinical measures (NPH: r = –0.71, p = 0.005, r2 = 0.50; NPH gait: rs = –0.70, p = 0.005).
Discussion
Our study shows that corpus callosum size is related to neuropsychological and clinical dysfunction in idiopathic NPH. Two frontal parts of the corpus callosum, which mainly contain projections from frontal cortical areas, were the regions most related to the clinical and neuropsychological dysfunction in NPH. These findings are consistent with the frontal involvement reported in the clinical deterioration of patients with NPH.15
To our knowledge, no previous studies have related corpus callosum thinning with neuropsychological functioning in adult NPH. The association between corpus callosum integrity and cognitive status found is in line with previous findings in other neurological conditions,11,16,17,18 normal ageing,19 and in children with congenital hydrocephalus,20,21 where corpus callosum size was markedly correlated with deficits in non‐verbal cognitive deficiencies. Damage to the corpus callosum has also been related to gait disturbances in patients with hydrocephalus,2 leucoaraiosis11,16 and callosal infarction.22 In a recent study, Mazza et al23 reported that patients undergoing sagittal callosotomy in third ventricle surgery showed a marked deficit in selective attention for the left visual field compared with patients receiving transverse callosotomy, a variant procedure which spares a larger number of callosal fibres.
In hydrocephalus, axonal degeneration is the major pathological feature.24 White matter destruction is due to a combination of mechanical injury (stretch), impaired blood flow, and accumulation of waste products in the cerebrospinal fluid.25,26 Callosal damage is probably a consequence of injury in and around the corpus callosum. Direct callosal injury is caused by ventricular compression and impingement against the falx,1,2 and secondary callosal damage is a result of periventricular white matter damage that causes axonal loss and Wallerian degeneration.27,28
This study shows that corpus callosum size increases markedly 6 months after surgery. This increase could reflect the re‐expansion effects after surgery, and corroborates the study of Röricht et al4 study, which reported normalisation of the area and shape of the corpus callosum 7 days after surgery in five shunted cases. Segev et al5 reported only a partial change in midline morphology (distances and angles between midline structures) towards normality. Occasionally, a generalised thickening or a slightly swollen appearance of the corpus callosum, in association with signal abnormality, has also been reported after surgery.7,8,10 In these studies, callosal abnormalities did not correlate with clinical data.
In our study, postsurgical corpus callosum increases in three regions were related to less improvement in several neuropsychological and clinical measures. The direction of these correlations was unexpected. A possible explanation for these results may be that when more expansion/decompression of the corpus callosum occurs, the hydrocephalus and the axonal damage are more severe, and hence less improvement is achieved.
Despite the multiple contrasts made and the risk of increasing error type I, we show the CI and effect sizes for each contrast for the parametric statistics. In most cases, moderate to high effect sizes were observed.
In conclusion, the structure–function relationship observed here shows that corpus callosum atrophy in patients with NPH is associated with cognitive and clinical dysfunction, supporting the notion that corpus callosum abnormalities reflect the cerebral damage produced by the illness. This study also shows that corpus callosum size increases 6 months after shunt surgery, but these increases seem to be related to poorer neuropsychological and clinical outcome. The corpus callosum increase may be the result of decompression, re‐expansion or an increase in interstitial fluid, or it may be caused by differences in shape due to cerebral reorganisation. Further volumetric studies using voxel‐based morphometry techniques can elucidate whether this effect is due to differences in shape.
Abbreviations
MRI - magnetic resonance imaging
NPH - normal pressure hydrocephalus
Footnotes
Funding: This study was partially supported by grants FIS 99/0968 and 2001 SGR 00139.
Competing interests: None.
References
- 1.El Gammal T, Allen M B, Brooks B S.et al MR evaluation of hydrocephalus. Am J Roentgenol 1987149807–813. [DOI] [PubMed] [Google Scholar]
- 2.Jinkins J R. Clinical manifestations of hydrocephalus caused by impingement of the corpus callosum on the falx: an MR study in 40 patients. Am J Neuroradiol 199112331–340. [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman E, Becker T, Jackel M.et al The corpus callosum in communicating and noncommunicating hydrocephalus. Neuroradiology 199537212–218. [DOI] [PubMed] [Google Scholar]
- 4.Röricht S, Meyer B U, Woiciechowsky C.et al Callosal and corticospinal tract function in patients with hydrocephalus: a morphometric and transcranial magnetic stimulation study. J Neurol 1998245280–288. [DOI] [PubMed] [Google Scholar]
- 5.Segev Y, Metser U, Beni‐Adani L.et al Morphometric study of the midsagittal MR imaging plane in cases of hydrocephalus and atrophy and in normal brains. Am J Neuroradiol 2001221674–1679. [PMC free article] [PubMed] [Google Scholar]
- 6.Numaguchi Y, Kristt D A, Joy C.et al Scalloping deformity of the corpus callosum following ventricular shunting. Am J Neuroradiol 199314355–362. [PMC free article] [PubMed] [Google Scholar]
- 7.Spreer J, Ernestus R I, Lanfermann H.et al Lesions of the corpus callosum in hydrocephalic patients with ventricular drainage: a CT‐study. Acta Neurochir 1996138174–178. [DOI] [PubMed] [Google Scholar]
- 8.Suh D Y, Gaskill‐Shipley M, Nemann M W.et al Corpus callosal changes associated with hydrocephalus: a report of two cases. Neurosurgery 199741488–494. [DOI] [PubMed] [Google Scholar]
- 9.Lane J I, Luetmer P H, Atkinson J L. Corpus callosal signal changes in patients with obstructive hydrocephalus after ventriculoperitoneal shunting. Am J Neuroradiol 200122158–162. [PMC free article] [PubMed] [Google Scholar]
- 10.Constantinescu C S, McConachie N S, White B D. Corpus callosum changes following shunting for hydrocephalus: case report and review of the literature. J Clin Neurol Neurosurg 2005107351–354. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi H, Fukuyama H, Ogawa M.et al Callosal atrophy in patients with lacunar infarction and extensive leukoaraiosis. An indicator of cognitive impairment. Stroke 1994251788–1793. [DOI] [PubMed] [Google Scholar]
- 12.Poca M A, Mataro M, Matarín M M.et al Is placement of shunts in patients with idiopathic normal‐pressure hydrocephalus worth the risk? Results of a study based on continuous monitoring of intracranial pressure. J Neurosurg 2004100855–866. [DOI] [PubMed] [Google Scholar]
- 13.Lezak M D, Howieson D B, Loring D W.Neuropsychological assessment. New York: Oxford University Press, 2004
- 14.Witelson S F. Hand and sex differences in the isthmus and genu of the human corpus callosum. Brain 1989112799–835. [DOI] [PubMed] [Google Scholar]
- 15.Iddon J L, Pickard J D, Cross J J.et al Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer's disease: a pilot study. J Neurol Neurosurg Psychiatry 199967723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretti M, Carlucci G, Di Carlo A.et al Corpus callosum atrophy is associated with gait disorders in patients with leukoaraiosis. Neurol Sci 20052661–66. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier J, Habib M, Lyon‐Caen O.et al Functional and magnetic resonance imaging correlates of callosal involvement in multiple sclerosis. Arch Neurol 1993501077–1082. [DOI] [PubMed] [Google Scholar]
- 18.Verger K, Junque C, Levin H S.et al Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Inj 200115211–221. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan E V, Pfefferbaum A, Adalsteinsson E.et al Differential rates of regional brain change in callosal and ventricular size: a 4‐year longitudinal MRI study of elderly men. Cereb Cortex 200212438–445. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher J M, Bohan T P, Brandt M E.et al Cerebral white matter and cognition in hydrocephalic children. Arch Neurol 199249818–824. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher J M, Bohan T P, Brandt M E.et al Morphometric evaluation of the hydrocephalic brain: relationships with cognitive development. Childs Nerv Syst 199612192–199. [DOI] [PubMed] [Google Scholar]
- 22.Giroud M, Dumas R. Clinical and topographical range of callosal infarction: a clinical and radiological correlation study. J Neurol Neurosurg Psychiatry 199559238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazza M, Di Rienzo A, Costagliola C.et al The interhemispheric transcallosal‐transversal approach to the lesions of the anterior and middle third ventricle: surgical validity and neuropsychological evaluation of the outcome. Brain Cogn 200455525–534. [DOI] [PubMed] [Google Scholar]
- 24.Ding Y, McAllister J P I I, Yao B.et al Axonal damage associated with enlargement of ventricles during hydrocephalus: a silver impregnation study. Neurol Res 200123581–587. [DOI] [PubMed] [Google Scholar]
- 25.Del Bigio M R. Neuropathological changes caused by hydrocephalus. Acta Neuropathol 199385573–585. [DOI] [PubMed] [Google Scholar]
- 26.Del Bigio M R, Wilson M J, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol 200353337–346. [DOI] [PubMed] [Google Scholar]
- 27.Simon J H, Schiffer R B, Rudick R A.et al Quantitative determination of MS‐induced corpus callosum atrophy in vivo using MR imaging. Am J Neuroradiol 19878599–604. [PMC free article] [PubMed] [Google Scholar]
- 28.Leys D, Pruvo J P, Parent M.et al Could Wallerian degeneration contribute to leuko‐araiosis in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry 19915446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]