Abstract
Background
Alzheimer's disease (AD) and frontotemporal dementia (FTD) are the commonest causes of presenile dementia. In the absence of a biological marker, diagnosis is reliant on clinical evaluation. Confirmation is often sought from neuroimaging, including single‐photon emission computed tomography (SPECT). Most previous SPECT studies lack pathological validation.
Aim
To examine the accuracy of SPECT in differentiating FTD from AD in patients with subsequent pathological confirmation.
Methods
Technetium‐99‐labelled hexamethyl propylene amine oxime SPECT images obtained at initial evaluation in 25 pathologically confirmed cases of FTD were examined. These images were visually rated by an experienced blinded nuclear medicine consultant and compared with those of 31 patients with AD, also with pathological validation.
Results
A reduction in frontal cerebral blood flow (CBF) was more common in FTD and was of diagnostic value (sensitivity 0.8, specificity 0.65 and likelihood ratio (LR) 2.25; 95% CI 1.35 to 3.77). A pattern of bilateral frontal CBF reduction without the presence of associated bilateral parietal CBF change is diagnostically more accurate (sensitivity 0.80, specificity 0.81 and +LR 4.13, 95% CI 1.96 to 8.71). Diagnostic categorisation (FTD or AD) on the basis of SPECT alone was less accurate than clinical diagnosis (based on neurology and detailed neuropsychological evaluation). One patient with FTD was initially clinically misdiagnosed as AD, owing to the lack of availability of full neuropsychological assessment. However, SPECT correctly diagnosed this patient, providing a diagnostic gain of 4%.
Conclusion
Technetium‐99‐labelled hexamethyl propylene amine oxime SPECT CBF patterns provide valuable information in the diagnosis of FTD and AD. These data can be better used as an adjunct to clinical diagnosis if pathology is to be correctly predicted in life.
Frontotemporal dementia (FTD) is a cortical dementia distinct from other dementing illnesses. It typically presents with personality/behavioural change and decline in social conduct with early loss of insight.1,2 In the absence of biological markers, the pathological detection of characteristic histological changes remains the gold standard of diagnosis. In life, diagnosis is primarily based on patterns of neurological and neuropsychological findings. However, differentiation from other dementias can be difficult and demands an astute qualitative analysis of various behaviours and neuropsychological test performances.3 With a paucity of experienced neuropsychologists, additional and independent diagnostic information is often sought through imaging, be it structural (CT and MRI) and/or functional (single‐photon emission computed tomography (SPECT) and positron emission tomography).
SPECT is used to evaluate patients with dementia and can show purported characteristic changes in FTD and in Alzheimer's disease (AD).4,5,6,7,8,9 The technique provides a method of evaluating blood flow in various regions of the brain, which reflects areas of poor function by showing reductions in regional cerebral blood flow (CBF). It has been shown that posterior changes in regional CBF are common in AD,4,5,6,7 whereas in FTD anterior changes are prevalent7,8,9 and posterior changes rare.7
However, CBF changes are neither wholly specific nor invariable in various dementing illnesses. Masterman et al10 looked at the value of bitemporal hypoperfusion in diagnosing AD, and found that, although a sensitive measure for detecting dementia (0.75), it was poorly specific for AD (0.55). Consequently, bitemporal hypoperfusion on SPECT can be a non‐specific finding in various forms of dementia and is not exclusive to AD. Starkstein et al11 reported deficits in CBF in the frontal (especially orbitofrontal) and anterior temporal cortices in FTD. However, they provided neither the measure of the diagnostic accuracy of SPECT in FTD nor of the diagnostic gain it may provide. Most of these studies are also limited by the fact that the dementia groups are defined clinically. The clinical diagnostic accuracy of FTD in life varies hugely between 14–85%.12,13,14
A few studies have looked at the accuracy of clinical and SPECT findings in relation to the final pathological diagnoses.15,16,17,18,19 Although these studies found that SPECT findings do correlate with dementia type, they failed to enquire whether SPECT provides any additional diagnostic gain over clinical judgement. These studies are also severely limited by the small numbers of patients in the FTD groups.
The aims of this study include evaluation of the diagnostic accuracy of SPECT in differentiating FTD from AD at initial assessment in a group of patients with final pathological confirmation of diagnosis. We also examined the diagnostic gain SPECT may provide over clinical diagnosis of FTD from among this group of patients with FTD and AD.
Methods
Patient selection
The study group comprised 43 patients in whom the pathological diagnosis was FTD. These patients had been seen at our tertiary referral centre between 1985 and 1998, and have been fully characterised pathologically in Shi et al.20 Twenty‐five SPECT scans were available for analysis.
The control group comprised 31 patients with AD, pathologically confirmed using the Consortium to Establish a Registry for AD criteria for the neuropathological diagnosis.21 These patients, in whom SPECT images were available for analysis, had been assessed during the same period as the study group. In both groups, consent for postmortem examination had been obtained during life and confirmed at death. This study is part of a larger research programme and ethical approval has been obtained.
Clinical diagnosis at first presentation
The clinical records of both FTD and AD groups were scrutinised. These patients had been assessed longitudinally using detailed neurological and neuropsychological evaluation developed at our centre (Greater Manchester Neurosciences Center, Salford, UK).22 The patients had been assessed in detail, including a cognitive and behavioural history, clinical neurological examination and neuropsychological assessment. This neuropsychological examination included observation of behaviour and cognitive testing of various domains including memory, language, visuospatial function, perception, praxis, executive function and attention. This assessment included a qualitative analysis of various test failures in addition to their quantitative evaluation. The overall clinical diagnosis had been based on a consensus between the neurologist and neuropsychologist in a multidisciplinary setting. This presumptive clinical diagnosis, made at first presentation, was before SPECT scanning was recorded. Patients had been followed up every 6 months until their death.
SPECT scanning
SPECT scans were obtained by injecting 500 MBq technetium‐99‐labelled hexamethyl propylene amine oxime (Amersham, Bucks, UK), with the patients seated in quiet surroundings with eyes open. Data were acquired and reconstructed using a Toshiba GCA‐901A/SA (Toshiba Medical Systems, Tokyo, Japan) integrated digital camera and computer system, using a single rotating detector fitted with a low‐energy high‐resolution collimator. Sixty 20 s views over a 360° elliptical orbit were obtained. The raw data were corrected for uniformity variation and then smoothed using an 11×11 preprocessing filter. Transaxial sections were produced using filtered back projections. These were then corrected for γ ray attenuation, using the Chang method for an attenuated coefficient of 0.1/cm.23 The data were reformatted to provide transaxial slices of 2 pixel (8 mm) thickness to the orbitomeatal line. Transaxial, coronal and parasagittal sections were reconstructed.24 SPECT scans, performed within 1 month of the first clinical assessment, were included in the study.
Rating of CBF
The images were reported by a consultant (MM) in nuclear medicine, experienced in the interpretation of SPECT images of patients with dementia. They were reported blind to all clinical and pathological data. The scans were rated 0 or 1 for normal or abnormal CBF, respectively. This rating was performed regionally for frontal, parietal, temporal and occipital regions on the left and right. Asymmetry was rated as either absent or present (0 and 1, respectively). Blood flow was assessed using a coloured magenta heat scale. Areas were considered abnormal if they were below the halfway point of this scale on more than two sections. Finally, a diagnosis was made using a choice of FTD, AD or “non‐specific”.
To validate the visual rating method, a second consultant (HJT) rated 16 SPECT scans drawn at random.
Statistical analysis
SPSS V.10 was used for data analysis. Continuous variables such as age and duration of illness were analysed using analysis of variance, whereas Pearson's χ2 test or Fisher's exact test was used to treat non‐parametric categorical data. The assessment of the incremental diagnostic value of SPECT over and above clinical diagnosis and SPECT diagnosis alone was performed using a forward logistic regression model. Significance value was set at p = 0.05.
Inter‐rater reliability was calculated using Cohen's κ statistic of agreement.
Results
Patient demographics
There were no significant differences in the mean (SD) age at initial assessment and duration of illness between the groups. Males outnumbered females in both groups. The Mini Mental State Examination (MMSE) score was significantly lower in the AD group (table 1).
Table 1 Patient demographics.
| Group (n) | Mean (SD) age (years) | Sex M:F | Mean (SD) duration (years) | Mean (SD) MMSE |
|---|---|---|---|---|
| FTD (25) | 58 (10) | 18:7 | 4 (4) | 20 (7)* |
| AD (31) | 61 (7) | 20:11 | 4 (2) | 16 (6)* |
AD, Alzheimer's disease; F, Female; FTD, frontotemporal dementia; M, male; MMSE, Mini Mental State Examination.
*p<0.05.
SPECT findings
Inter‐rater reliability
The overall κ agreement for all the rated regions was 0.68. The agreement between the two raters for the diagnosis based on SPECT alone (without clinical information) was moderate (κ 0.48).
Regional CBF in FTD and AD
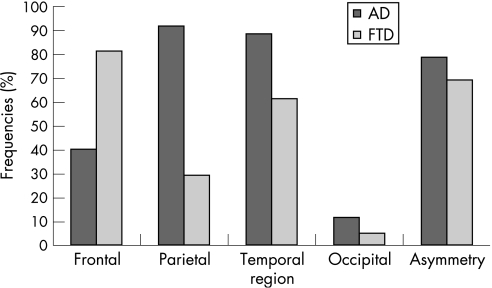
Table 2 and figure 1 show the results for the regional CBF changes in FTD and AD. Significant differences were seen in frontal, parietal and temporal regions. There were no differences in the CBF in occipital regions. The frequency of asymmetry between the two groups was also not significantly different. In all, 80% of the patients with FTD showed reduction in frontal CBF. The majority (72%) of patients with FTD showed no changes in parietal CBF. In all, 90% of patients with AD showed reduced parietal CBF (table 2).
Table 2 Regional cerebral blood flow change on single‐photon emission computed tomography in frontotemporal dementia and Alzheimer's disease.
| Regional rating | FTD (n = 25) n (%) | AD (n = 31) n (%) | FTD vs AD, p value | |
|---|---|---|---|---|
| Frontal | 0 | 5 (20) | 19 (61) | |
| 1 | 20 (80) | 12 (39) | 0.002** | |
| Parietal | 0 | 18 (72) | 3 (10) | |
| 1 | 7 (28) | 28 (90) | 0.000** | |
| Temporal | 0 | 10 (40) | 4 (13) | |
| 1 | 15 (60) | 27 (87) | 0.02* | |
| Occipital | 0 | 24 (96) | 25 (81) | |
| 1 | 1 (4) | 6 (11) | 0.12 | |
| Asymmetry | 0 | 8 (32) | 7 (23) | |
| 1 | 17 (68) | 24 (78) | 0.43 | |
AD, Alzheimer's disease; FTD, frontotemporal dementia.
*p<0.05
**p<0.01
Figure 1 Frequencies (%) of reduced regional cerebral blood flow (CBF) change in frontotemporal dementia (FTD) and Alzheimer's disease (AD).
FTD versus non‐FTD (AD)
Frontal CBF reduction increased the likelihood of diagnosis of FTD (sensitivity 0.8, specificity 0.65 and LR 2.25; 95% CI 1.35 to 3.77; table 3). A pattern of only bilateral frontal change, without associated bilateral parietal change, was even more specific for FTD (sensitivity 0.8, specificity 0.81 and LR 4.13, 95% CI 1.96 to 8.71). Temporal CBF changes on SPECT were not impressive in their discrimination of FTD from AD. However, parietal change in CBF was a strongly negative predictor of FTD (LR 0.31, 95% CI 0.16 to 0.59).
Table 3 Diagnostic sensitivity, specificity and likelihood ratios of change in regional cerebral blood flow.
| Region | FTD vs non‐FTD (AD) | AD vs non‐AD (FTD) | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | +LR(CI) | Sensitivity | Specificity | +LR(CI) | |
| Predominant bifrontal plus† | 0.8 | 0.65 | 2.25 (1.35 to 3.77)* | 0.61 | 0.12 | 0.7 (0.27 to 0.74)* |
| Predominant parietal plus‡ | 0.28 | 0.1 | 0.31 (0.16 to 0.59)* | 0.9 | 0.72 | 3.23 (1.7 to 6.11)* |
| Temporal | 0.6 | 0.13 | 0.69 (0.49 to 0.98)* | 0.87 | 0.4 | 1.45 (1.03 to 2.05)* |
| Only bifrontal | 0.8 | 0.81 | 4.13 (1.96–8.71)* | |||
| Only parietal | 0.58 | 1 | ∞* | |||
AD, Alzheimer's disease; FTD, frontotemporal dementia; LR, likelihood ratio.
*CI significantly different from 1.
†Dominant bifrontal change with/without other regional cerebral blood flow change.
‡Dominant parietal change with/without other regional cerebral blood flow change.
SPECT diagnosis versus clinical diagnosis
Table 4 compares the diagnostic value of both clinical and SPECT methods. In both disease groups, the clinical diagnosis (defined in Methods section) was found to have a higher diagnostic value than blinded SPECT diagnosis. The SPECT diagnoses for both FTD and AD improved significantly with the aid of clinical history (table 4).
Table 4 Sensitivity, specificity and likelihood ratios of clinical diagnosis and single‐photon emission computed tomography.
| Diagnostic tool | FTD vs non‐FTD (AD) | AD vs non‐AD (FTD) | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | +LR (CI) | Sensitivity | Specificity | +LR(CI) | |
| Clinical† | 0.88 | 0.77 | 3.9 (2 to 7.6)* | 0.77 | 0.88 | 6.45 (2.19 to 18.97)* |
| SPECT alone | 0.72 | 0.65 | 2.23 (1.27 to 3.93)* | 0.65 | 0.72 | 2.3 (1.17 to 4.55)* |
| SPECT+clinical | 0.84 | 0.84 | 5.24 (2.11 to 13.04)* | 0.84 | 0.84 | 5.24 (2.11 to 13)* |
AD, Alzheimer's disease; FTD, frontotemporal dementia; LR, likelihood ratio; SPECT, single‐photon emission computed tomography.
*CI significantly different from 1.
†Clinical diagnosis including neurological and neuropsychological evaluation at first presentation.
Additional value of SPECT over initial clinical diagnosis
The forward stepwise logistic regression model was fit using initial clinical diagnosis alone, SPECT diagnosis alone, and then with the addition of SPECT diagnosis to clinical diagnosis to determine what extra information, if any, is gained (table 5). This model demonstrates that clinical diagnosis is a better predictor of pathological diagnosis than SPECT in isolation. In the group with FTD, SPECT correctly diagnosed 72% of the patients. However, clinical diagnosis was able to detect 88% of patients with FTD. SPECT helped to improve detection of FTD by an additional 4%, thus correctly diagnosing 92% of the patients when combined with the clinical diagnosis.
Table 5 Additional value provided by diagnosis using single‐photon emission computed tomography.
| Diagnostic modality | Percentage of correct diagnoses | ||
|---|---|---|---|
| FTD | AD | Overall | |
| Clinical diagnosis alone | 88 | 90.3 | 89.3 |
| SPECT diagnosis alone | 72 | 87.1 | 80.4 |
| Clinical+SPECT | 92 | 90.3 | 91.1 |
AD, Alzheimer's disease; FTD, frontotemporal dementia; SPECT, single‐photon emission computed tomography.
Misdiagnosis
Three patients in the group with FTD were misdiagnosed clinically at their first presentation, in two because of the absence of behavioural and executive deficits, and in one due to lack of availability of full neuropsychological assessment. In this latter case, the SPECT had shown a bifrontal deficit. In the group with AD, seven patients were misdiagnosed. Three had an atypical frontal presentation, both clinically and on SPECT. One patient presented with language disorder, two with focal neurological signs and one with fluctuating confusional state. The SPECT was recorded as non‐specific in these patients.
Discussion
Over the past decade, accurate diagnosis of dementias in life has become increasingly important with the availability of current modern therapies and promise of future novel treatments. Clinical diagnosis is often a reflection of clinical expertise and experience. Increased diagnostic precision is often sought from neuroimaging, including SPECT scanning.17 FTD is a common form of dementia and is often misdiagnosed and commonly confused with AD.12,25 Although SPECT is thought to be helpful in differentiating FTD from AD,26 there are very few studies which comment on the accuracy of this technique against the gold standard of diagnosis—that is, pathological verification. Moreover, it is not known whether SPECT alone is sufficient to make a diagnosis of FTD, or whether it provides any additional value over clinical diagnosis. This study evaluated the utility of SPECT in differentiating FTD from AD in a group of patients with subsequent pathological confirmation of diagnosis. We also examined the clinical utility of SPECT in diagnosing FTD in clinical practice.
This study confirms previous SPECT findings of frontal CBF reduction in FTD.9,11,27,28 A pattern of frontal CBF hypoperfusion increases the probability of a patient having FTD.24,28 However, these conclusions are limited by the lack of pathological authentication of diagnosis in the patient groups studied. By contrast, previous postmortem studies have not assessed the diagnostic value of distinct patterns of SPECT findings. Our study is strengthened by the larger number of patients with FTD and the optimal analysis of data (tables 1, 3–5), thus evaluating and providing a measure of the diagnostic potential of SPECT. Frontal CBF reduction significantly increases the likelihood of FTD, whereas parietal hypoperfusion significantly decreases the likelihood of FTD (table 3, fig 2). The sensitivities and specificities found in our work (table 3) are similar to those described in other postmortem studies.15,16,18 However, these studies are restricted by the small numbers of patients studied in the non‐AD group. For example, although Jobst et al16 included 24 patients with alternative clinical diagnoses to AD, the FTD group comprised only three patients. Similarly, Jagust et al18 studied only 14 patients without AD, of whom only two had FTD. The authors comment that their sample is insufficient to evaluate the use of SPECT in the differential diagnosis of dementia. Our study, therefore, crucially addresses these inadequacies by studying FTD in sufficient numbers, and then evaluating the role of SPECT in detecting FTD from within a group of patients with and without FTD (AD) with subsequent pathological confirmation.
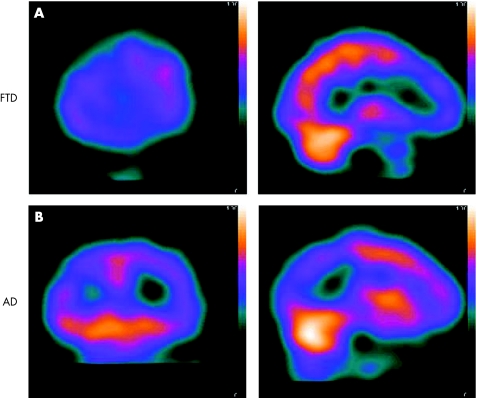
Figure 2 (A) Frontotemporal dementia (FTD): coronal and sagittal single‐photon emission computed tomography (SPECT) images showing reduced cerebral blood flow (CBF) in frontal regions. (B) Alzheimer's disease (AD): coronal and sagittal SPECT images showing reduced CBF in parietal regions.
SPECT in isolation was less accurate than the diagnosis provided by comprehensive clinical (including neuropsychological) evaluation for both FTD and AD (table 5). Therefore, the clinical diagnosis in a specialist unit is a better predictor of the pathological diagnosis. However, the addition of SPECT to clinical diagnosis results in improved prediction of the pathological diagnosis (table 5). Interestingly, SPECT did not provide any additional information to the clinical diagnosis in patients with AD. The patients with AD misdiagnosed as FTD were atypical in the frontal clinical presentation and SPECT changes. However, SPECT did increase the percentage of patients correctly diagnosed as having FTD from 88% (clinical diagnosis) to 92% (clinical diagnosis with SPECT). One patient with FTD with an initial misdiagnosis of AD was correctly diagnosed subsequently after full neuropsychological testing. SPECT failed to diagnose 28% of patients with FTD and 13% of patients with AD (table 5). These findings argue against using SPECT as the diagnostic tool, in isolation, for the differentiation of FTD and AD. Consequently, SPECT is optimally used as an adjunct to detailed clinical, including neuropsychological, analysis.
To provide further support for this argument, it is relevant to discuss the SPECT images that were initially diagnosed incorrectly. The same nuclear medicine physician (MM) rated the images for a second time, after being given the clinical history. This information increased the diagnostic accuracy of SPECT with improved sensitivity (0.84), specificity (0.84) and LR ratio (5.24, 95% CI 2.11 to 13.04) (table 4). It is notable that regional CBF change is reliably detected by independent raters (κ 0.68); however, the agreement for the overall diagnosis based on the interpretation of these SPECT findings was moderate (κ 0.48). Correct diagnostic categorisation of SPECT scans can be enhanced both with experience and by ensuring that the interpretation is made with pertinent clinical information.
To the best of our knowledge, this is the first study to evaluate the additional value of SPECT in detecting FTD from within a group of dementias with pathological definition. Jagust et al18 did show that SPECT contributes to the diagnosis of AD by improving the diagnostic yield of AD pathology by 7–8%, increasing the specificity to a value of >90% on addition of SPECT to clinical assessment. However, AD is very common in the population; thus, patients presenting to a dementia clinic are far more likely to be suffering from AD than other less prevalent forms of dementia. The diagnostic gain of SPECT, therefore, may be more useful in less common dementias such as FTD. Our study suggests that SPECT provides a diagnostic increase of 4% over clinical diagnosis. This gain refers to the single patient in whom adequate neuropsychology was not available at presentation. Neuropsychology is an essential tool in differentiating dementias.3 However, such services are not widely available. Consequently, SPECT is likely to be of even greater benefit in a less specialised setting where neuropsychological assessment is not routinely available. Repetition of this study in a non‐specialist setting may help clarify this point further, although the small number of patients with FTD in such situations is likely to limit such an endeavour.
Our study clearly demonstrates that SPECT alone is specific neither for FTD nor for AD (table 4). Jagust et al's18 findings are comparable in their patients with pathological verification. However, Charpentier et al29 claim that MMSE and SPECT scanning alone may provide a correct diagnosis of FTD in 100% of patients. These patients were not pathologically defined, and the interpretation of SPECT scans does not seem to be blinded to clinical diagnosis. Our findings caution against such conclusions and suggest that SPECT is not an infallible tool in the diagnosis of FTD. A more appropriate role for SPECT is as an adjunct to the clinical diagnosis of FTD.
This study has been carried out in a group of patients with young‐onset FTD and AD. The lower mean ages reflect a referral bias at our specialist centre and older patients may be more likely to be referred to geriatric services than to a neurology department. In Jagust et al's18 study, 66% of patients with FTD were clinically misdiagnosed with AD, with a mean age of 77.1 years. FTD is a disorder seen mainly in the presenium, and in a younger population FTD may comprise up to 20% of dementia.2 Consequently, a percentage of misdiagnosis similar to that by Jagust et al18 would reflect numerically larger numbers in a younger cohort. Therefore, it is all the more relevant to scrutinise the diagnostic differential of FTD in populations comparable to that of our study. The large number of appropriate patients studied, the pathological gold standard of diagnosis and the individual and added values of various diagnostic methods enhance the value of our findings.
The severity of dementia in our study group was mild to moderate at the time of first assessment and scan, as shown by the mean duration of illness and the MMSE scores (table 1). The mean MMSE score was significantly lower in the AD group reflecting the prominent memory deficits in this condition. The higher scores in the FTD group highlight a bias of the MMSE to deficits that, although commonly seen in other dementias, are rare in FTD. Therefore, our study demonstrates the utility of SPECT in the diagnosis of mild to moderate dementia, in comparison with other studies18 that were evaluated at a more severe stage.
It is relevant to discuss the limitations of our study. A specialist centre does not reflect the disease seen in primary or secondary care. Our diagnostic methods are thus not universally comparable. We opted to use patients with AD as the control group because such a comparison is more clinically relevant than a simple contrast with a normal control population. In clinical practice, one is usually faced with the question of differentiating one form of dementia from the other rather than from non‐demented normal subjects without dementia. It may also be argued that our study used visual assessment of SPECT rather than semiquantitive techniques, which might have led to a less accurate measure of CBF. However, previous studies have shown that semiquantitive techniques offer no additional benefit over visual ratings.15 Furthermore, in routine clinical practice, visual assessment of SPECT is the diagnostic method most often used and has been previously validated.28 Consequently, this study is of relevance to common clinical practice and indeed fulfils its primary aim of evaluating the practical role of SPECT in helping to detect FTD and differentiate it from AD.
In conclusion, this study clearly shows that SPECT should not be used in isolation for the diagnosis of FTD. We have also demonstrated and quantified the usefulness of SPECT in detecting FTD by providing a measure of the diagnostic gain added to clinical diagnosis at our centre. The appropriate diagnostic role for SPECT, therefore, is as an adjunct to the clinical diagnosis of FTD. This study also raises the possibility of an important role for SPECT in increasing diagnostic precision outside specialised settings.
Abbreviations
AD - Alzheimer's disease
CBF - cerebral blood flow
FTD - frontotemporal dementia
MMSE - Mini Mental State Examination
SPECT - single‐photon emission computed tomography
Footnotes
Competing interests: None declared.
References
- 1.Neary D, Snowden J S, Gustafson L.et al Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 199851546–554. [DOI] [PubMed] [Google Scholar]
- 2.Snowden J, Neary D, Mann D. Fronto‐temporal dementia. In: Frontotemporal lobar degeneration: frontotemporal dementia, progressive aphasia, semantic dementia Edinburgh: Churchill Livingstone, 19969
- 3.Snowden J. Neuropsychological evaluation and the diagnosis and differential diagnosis of dementia. Rev Clinl Gerontol 1999965–72. [Google Scholar]
- 4.Neary D, Snowden J, Shields R.et al Single photon emission tomography using 99mTc‐HMPAO in the investigation of dementia. J Neurol Neurosurg Psychiatry 1987501101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns A, Philpot M P, Costa D C.et al The investigation of Alzheimer's disease with single photon emission computerised tomography. J Neurol Neurosurg Psychiatry 198952248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith I G, Bartholomew P H, Irvine E M. Single photon emission computerised tomography in elderly patients with Alzheimer's disease and multi‐infarct dementia. Regional uptake of technetium‐labelled HMPAO related to clinical measurement. Br J Psychiatry 1993163597–603. [DOI] [PubMed] [Google Scholar]
- 7.Talbot P, Snowden J, Lloyd J.et al The contribution of single photon emission tomography to the clinical differentiation of degenerative cortical brain disorders. J Neurol 1995242579–586. [DOI] [PubMed] [Google Scholar]
- 8.Miller B, Cummings J, Villanueva‐Meyer J.et al Frontal lobe degeneration: clinical, neuropsychological and SPECT characteristics. Neurology 1991411374–1381. [DOI] [PubMed] [Google Scholar]
- 9.Risberg J. Frontal lobe degeneration of non‐Alzheimer type. III. Regional cerebral blood flow. Arch Gerontol Geriatr 19876225–233. [DOI] [PubMed] [Google Scholar]
- 10.Masterman D, Mendez M, Fairbanks L. Sensitivity, specificity and positive predictive value of Technetium 99‐HMPAO SPECT in discriminating Alzheimer's disease from other dementias. J Geriatr Psychiatr Neurol 19971015–21. [DOI] [PubMed] [Google Scholar]
- 11.Starkstein S, Migliorelli R, Teson A.et al Specificity of changes in cerebral blood flow in patients with frontal lobe dementia. J Neurol Neurosurg Psychiatry 199457790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez M F, Selwood B A, Mastri A R.et al Pick's disease versus Alzheimer's disease: a comparison of clinical characteristics. Neurology 199343289–292. [DOI] [PubMed] [Google Scholar]
- 13.Litvan I, Agid M D, Sastrj B S.et al What are the obstacles for an accurate clinical diagnosis of Pick's disease? A clincopathologic study. Neurology 19974962–69. [DOI] [PubMed] [Google Scholar]
- 14.Knopman D S, Boeve B S, Parisi J E.et al Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 200557480–488. [DOI] [PubMed] [Google Scholar]
- 15.Bonte F, Weiner M, Bigio E.et al Brain blood flow in the dementias: SPECT with histopathological correlation in 54 patients. Radiology 1997202793–797. [DOI] [PubMed] [Google Scholar]
- 16.Jobst K, Barnetson L, Shepstone B. Accurate prediction of histologically confirmed Alzheimer's disease and the differential diagnosis of dementia: the use of NINCDS–ADRDA and DSM‐III‐R criteria, SPECT, X‐ray CT and ApoE4 in medial temporal lobe dementias. Int Psychogeriatr 199810271–302. [DOI] [PubMed] [Google Scholar]
- 17.Read S, Miller B, Mena I.et al SPECT in dementia: clinical and pathological correlation. J Am Geriatr Soc 1995431243–1247. [DOI] [PubMed] [Google Scholar]
- 18.Jagust W, Thisted R, Devous M.et al SPECT perfusion imaging in the diagnosis of Alzheimer's disease: a clinical‐pathologic study. Neurology 200156950–956. [DOI] [PubMed] [Google Scholar]
- 19.Bonte F J, Harris T S, Hynan L S.et al Tc‐99m HMPAO SPECT in the differential diagnosis of the dementias with histopathological confirmation. Clin Nucl Med 200631376–378. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Shaw C L, Richardson A M T.et al Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol 2005110501–512. [DOI] [PubMed] [Google Scholar]
- 21.Mirra S S, Heyma A, McKeel D.et al The consortium to establish a registry for Alzheimer's disease. Part II. Standardisation of the neuropathologic assessment of Alzheimer's disease. Neurology 199141479–486. [DOI] [PubMed] [Google Scholar]
- 22.Neary D, Snowden J S, Bowen D M.et al Neuropsychological syndromes in presenile dementia due to cerebral atrophy. J Neurol Neurosurg Psychiatry 198649163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L T. A method of attenuation correction in radionucleotide computed tomograpgy. Trans Nucl Sci 197825638–643. [Google Scholar]
- 24.Talbot P, Lloyd J, Snowden J.et al A clinical role for 99mTc‐ HMPAO SPECT in the investigation of dementia? J Neurol Neurosurg Psychiatry 199864306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamer A J. Getting it wrong: the clinical misdiagnosis of Alzheimer's disease. Int J Clin Pract 2004581092–1094. [DOI] [PubMed] [Google Scholar]
- 26.Dougall N J, Bruggink S, Ebmeier K P. Systematic review of the diagnostic accuracy of 99mTc‐HMPAO‐SPECT in dementia. Am J Geriatr Psychiatry 20041254–70. [DOI] [PubMed] [Google Scholar]
- 27.Duara R, Barker W, Luis C A. Frontotemporal dementia and Alzheimer's disease: differential diagnosis. Dement Geriatr Cogn Disord 199910(Suppl 1)37–42. [DOI] [PubMed] [Google Scholar]
- 28.Varma A, Adams W, Lloyd J.et al Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer's disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand 2002105261–269. [DOI] [PubMed] [Google Scholar]
- 29.Charpentier P, Lavenu I, Defebvre L.et al Alzheimer's disease and frontotemporal dementia are differentiated by discriminant analysis applied to 99mTc HMPAO SPECT data. J Neurol Neurosurg Psychiatry 200069661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]