Abstract
Background
To assess whether the premorbid dietary intake of fatty acids, cholesterol, glutamate or antioxidants was associated with the risk of developing amyotrophic lateral sclerosis (ALS).
Methods
Patients referred to our clinic during 2001–2002, who had definite, probable or possible ALS according to El Escorial criteria, without a familial history of ALS, were asked to participate in a case–control study (132 patients and 220 healthy controls). A food‐frequency questionnaire was used to assess dietary intake for the nutrients of interest. Multivariate logistic regression analysis was performed with adjustment for confounding factors (sex, age, level of education, energy intake, body mass index and smoking).
Results
A high intake of polyunsaturated fatty acid (PUFA) and vitamin E was significantly associated with a reduced risk of developing ALS (PUFA: odds ratio (OR) = 0.4, 95% confidence interval (CI) = 0.2 to 0.7, p = 0.001; vitamin E: OR = 0.4, 95% CI = 0.2 to 0.7, p = 0.001). PUFA and vitamin E appeared to act synergistically, because in a combined analysis the trend OR for vitamin E was further reduced from 0.67 to 0.37 (p = 0.02), and that for PUFA from 0.60 to 0.26 (p = 0.005), with a significant interaction term (p = 0.03). The intake of flavonols, lycopene, vitamin C, vitamin B2, glutamate, calcium or phytoestrogens was not associated with the risk of developing ALS.
Conclusion
A high intake of PUFAs and vitamin E is associated with a 50–60% decreased risk of developing ALS, and these nutrients appear to act synergistically.
Sporadic amyotrophic lateral sclerosis (ALS) probably develops through the combined effects of several modifying genes and environmental factors.1 Despite several studies that investigated environmental exposures in relation to ALS, age, gender and smoking are the only established risk factors.2 Several, not mutually exclusive, pathological processes may contribute to motor neurone death in ALS in a so‐called convergence model,3 including oxidative stress, mitochondrial dysfunction, protein misfolding, axonal strangulation, apoptosis, inflammation, glutamate excitotoxicity and defects in neurotrophin biology. Nutrients are factors that could influence these processes and thereby the risk of developing ALS or its clinical expression.
ALS was previously found to be positively associated with intake of glutamate,4 fat,4 fish5 and milk,6,7 and inversely associated with intake of lycopene,8 dietary fibre,4 bread and pasta.9 Two other studies, however, failed to establish the relationship with milk.10,11 Several of these studies included only small samples of patients (<25),5,6,9 or investigated nutrition as one of many environmental factors, thus increasing the likelihood of chance findings.5,6,7,9,10,11 Furthermore, most studies did not account for the possible influence of clinical onset preceding the diagnosis5,6,7,8,9,10,11 or adjust for possible confounders including total energy intake, body mass index (BMI), sex, smoking and education.5,6,7,9,10,11
One study found an association between intake of total fat and ALS, although this was not hypothesised beforehand.4 This finding is of interest considering the observed associations of intake of saturated and unsaturated fatty acids and cholesterol with other neurodegenerative diseases.12 In this case–control study, therefore, we examined the possible association between premorbid dietary intake of fatty acids, cholesterol, glutamate, phytoestrogens, calcium and anti‐oxidants and the risk of developing ALS, adjusting for confounding factors.
Patients and methods
Patients
The University Hospitals in Amsterdam and Utrecht are national referral centres for ALS in The Netherlands. All patients included in this study were incident cases, who visited our clinics for diagnostic purposes during the 1‐year period between 2001 and 2002. Every patient who visited our clinic during this period and who had definite, probable or possible ALS according to El Escorial criteria,13 without a familial history of ALS, was asked to participate in the study. Because patients with suspected ALS may present a collection of other syndromes, only patients with both upper and lower motor neurone involvement were included.14 Accordingly, a total of 184 patients were identified and were sent a questionnaire, 132 (72%) of which were returned. Duration of disease, defined as the interval between onset of muscle weakness and death from any cause, tracheostomy or persistent assisted ventilation, was used for the survival analysis. The survival status of patients was monitored until May 2004. The 52 patients who did not return the questionnaire did not differ significantly from the 132 patients who did, with respect to sex, age at onset, duration of disease and type of onset.
Controls
Patients were sent three identical questionnaires. One questionnaire was to be completed by the patient and the other two by controls. Every patient was asked to approach two persons who fulfilled the following criteria:
should not be their spouse or partner
should preferably not differ from them in age by more than 5 years
should preferably be of the same sex.
Of 264 controls, 220 (83%) returned their questionnaires. All questionnaires remained anonymous, and all data were entered in a blinded fashion.
Questionnaire
The questionnaire was divided into two sections: the first section contained questions on age, sex, level of education, smoking and anthropometrical characteristics; the second section consisted of questions on food frequency. Patients were asked to recall their dietary habits during the period 1 year before the onset of muscle weakness or bulbar signs, to avoid a possible influence of subclinical disease on the factors of interest. Patients had to disclose this reference year to their controls, who had to report their dietary habits in the same reference year.
The food‐frequency questionnaire had 104 questions that were validated for the intake of total energy, total fat, fatty acids and cholesterol.15 The questionnaire was validated against linoleic acid concentrations in erythrocytes and adipose tissue as biomarkers of intake. These biomarkers were shown to be representative of long‐term intake of fatty acids, as the half‐life of linoleic acid in adipose tissue is approximately 680 days. The results of the questionnaire, therefore, are representative of a longer period of food intake. Considering the hypotheses of this study, the questionnaire was extended with questions on the dietary intake of glutamate, flavonols, lycopene, vitamin B2, vitamin C, vitamin E, calcium and phytoestrogens. Therefore, 37 items were added according to a systematic procedure. Food items for the original questionnaire were chosen on the basis of data from the Dutch National Food Consumption Survey of 1992.16 Data regarding vitamin C were also available from this survey, but other sources were used for the remaining nutrients: the national survey of 1987–1988;17 national reports by TNO Nutrition and Food Research and the Dutch Food Composition Tables (Nevo) for calcium, vitamin B2 and vitamin E; publications on flavonoids;18,19 the US Department of Agriculture table for phytoestrogens (isoflavones);20 publications on glutamate and monosodium glutamate;21,22,23,24,25 and the US Department of Agriculture table for lycopene.20 The 37 items that were added to the questionnaire on the basis of these sources accounted for at least 95% of the intake of a specific factor.
If questionnaires showed inconsistencies or if data were missing, the concerned were contacted by a nutritionist and asked for clarification. All questionnaires remained anonymous, and persons other than the nutritionist entered all the data in a blinded fashion.
Statistical analysis
Differences in categorical factors between patients and controls were determined by using the χ2 test. Differences in continuous variables were computed by using the Mann–Whitney U test (characteristics of patients and controls) and Student's t test (univariate nutrient comparison). To obtain nutrient data that were not correlated to total energy intake, the energy‐adjusted values were calculated according to the residual method.26 Nutrient data were categorised into tertiles based on the data of controls, and multivariate logistic regression was used to determine independent ORs for the association between intake of nutrients and ALS, the lowest tertile being the reference category. These three‐level variables were also entered into the model as continuous variables to determine whether there was a linear trend. The multivariate model always included the following possible confounders: sex, age (at onset for patients, current age for controls), level of education (low, middle, high), smoking (never, ever, current) and (premorbid) BMI.
If nutrients were markedly associated with ALS and biological interaction could be hypothesised, the interaction between these nutrients was tested by entering both nutrients and their product into the multivariate model.
We also carried out an exploratory analysis to test the possible association between clinical features (age at onset and duration of disease) and those nutrients that were independently associated with the risk of developing ALS. Cox regression analysis was used to determine the independent association between the intake of nutrients and age at onset of ALS adjusting for the above‐mentioned confounders and type of onset. The same procedure was used to determine the independent association between intake of nutrients and duration of disease, additionally adjusting for age at onset and type of onset.
All tests were two‐sided, and a p<0.05 was considered to be significant.
Results
Characteristics of patients and controls
Table 1 shows the characteristics of patients and controls, including the main potential confounding factors for the relationship between nutrient intake and ALS.
Table 1 Characteristics of patients with amyotrophic lateral sclerosis (ALS) and controls.
| Patients with ALS (n = 132) | Controls (n = 220) | |
|---|---|---|
| Age (years), median (range)* | 58 (25–79) | 59 (28–81) |
| Sex, n (%) | ||
| Male | 89 (67) | 136 (65) |
| Female | 43 (33) | 73 (35) |
| Site of onset, n (%) | ||
| Spinal | 105 (79) | |
| Bulbar | 27 (21) | |
| El Escorial category, n (%) | ||
| Possible | 29 (22) | |
| Probable | 79 (60) | |
| Definite | 24 (18) | |
| Education, n (%) | ||
| Low | 46 (35) | 64 (31) |
| Middle | 44 (33) | 62 (30) |
| High | 42 (32) | 82 (39) |
| Relationship with patient, n (%) | ||
| Friend | 106 (51) | |
| Direct family of partner | 65 (31) | |
| Neighbour | 11 (5) | |
| Partner | 8 (4) | |
| Direct family of patient | 8 (4) | |
| Therapist or care giver | 6 (3) | |
| Colleague | 4 (2) | |
| Smoking, n (%) | ||
| Never | 42 (32) | 60 (30) |
| Ever | 61 (46) | 101 (51) |
| Current† | 29 (22) | 39 (20) |
| Premorbid or current BMI, median, kg/m2 (range)‡ | 25 (18–50) | 25 (19–40) |
| Obese (>30) | 9 (7) | 11 (5) |
| Overweight (25–30) | 60 (46) | 89 (43) |
| Normal or underweight (<25) | 63 (48) | 108 (52) |
BMI, body mass index.
*Age at onset of disease for patients and current age for controls.
†For patients in the year before the onset of disease.
‡For patients, premorbid BMI; for controls, current BMI; in some cases, the sum of the data is not equivalent to the total of patients or controls, as some values are missing.
A representative sample of patients with ALS was obtained with respect to sex, although a younger age at onset and a relative predominance of patients with spinal onset in our sample suggest some referral bias as compared with a recent population‐based study.27
Patients and controls were not markedly different with regard to potential confounders (age, p = 0.21; sex, p = 0.66; education, p = 0.37; smoking, p = 0.73; BMI, p = 0.72). All analyses were adjusted for age and other potential confounding factors (gender, level of education, BMI and smoking).
Daily nutrient intake and risk of developing ALS
Table 2 compares the mean, energy‐adjusted daily intake of nutrients between patients and controls.
Table 2 Mean (SD) levels of premorbid daily nutrient intake in patients and controls.
| Patients with ALS patients (n = 132) | Controls (n = 220) | p Value | |
|---|---|---|---|
| Energy intake (MJ/day) | 12.3 (4.0) | 11.9 (4.3) | 0.40 |
| Total fat (g) | 135.2 (27.6) | 140.2 (30.2) | 0.12 |
| Saturated fat (g) | 51.2 (10.5) | 50.0 (8.6) | 0.24 |
| Monounsaturated fat (g) | 49.8 (17.2) | 52.5 (28.1) | 0.33 |
| Polyunsaturated fat (g) | 25.5 (10.5) | 29.3 (12.0) | 0.003 |
| Cholesterol (mg) | 314 (75) | 297 (91) | 0.07 |
| Flavonols (mg) | 24.4 (19.5) | 27.1 (19.1) | 0.20 |
| Lycopene (mg) | 4.6 (3.5) | 4.5 (3.6) | 0.68 |
| Glutamate (mg) | 826 (382) | 804 (347) | 0.58 |
| Vitamin C (mg) | 133 (69) | 145 (78) | 0.16 |
| Vitamin E (mg) | 17.8 (7.1) | 20.5 (9.2) | 0.004 |
| Riboflavin (mg) | 2.0 (0.5) | 2.0 (0.5) | 0.78 |
| Calcium (mg) | 1223 (452) | 1195 (383) | 0.53 |
| Phytoestrogens (mg) | 0.34 (1.3) | 0.89 (4.3) | 0.15 |
| Dietary supplements n (%) | 43 (33) | 78 (36) | 0.58 |
Values are energy adjusted according to the residual method.
Premorbid total energy intake was similar in the two groups, which is in accordance with similar premorbid BMI levels (table 1). Univariate analysis of total cholesterol intake showed a higher intake in patients with ALS, although this difference did not reach significance. Intake of polyunsaturated fatty acid (PUFA) and vitamin E was noticeably lower in patients with ALS than in controls. Premorbid daily intake of dietary supplements (mostly combinations of several nutrients) was also assessed, but did not differ significantly between patients and controls.
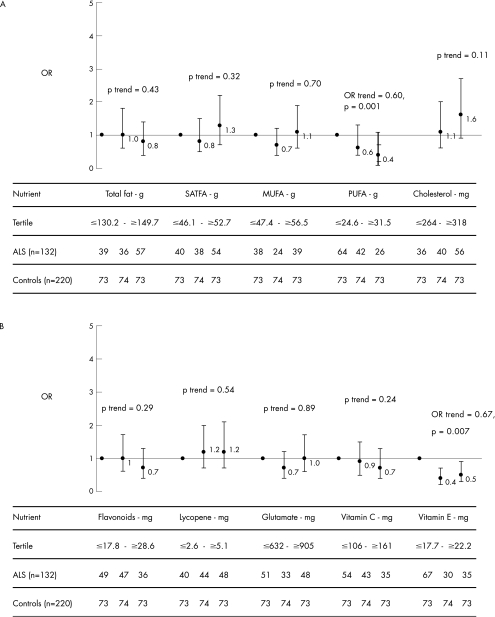
Figure 1 shows the adjusted ORs for the relationship between energy‐adjusted intake of nutrients and ALS. Multivariate analysis showed that the inverse association of intake of PUFAs and vitamin E with ALS was again highly significant. The highest tertile of daily PUFA intake (>32 g) was associated with a 60% lower risk of ALS compared with the lowest tertile (<25 g). The second tertile of vitamin E (18–22 mg) was associated with a 60% lower risk of ALS; the highest tertile of vitamin E intake (>22 mg) was associated with a 50% lower risk of ALS compared with the lowest tertile (<18 mg). The association with cholesterol intake was not significant (highest tertile compared with the lowest tertile OR = 1.6, 95% CI = 0.9 to 2.7, p = 0.11).
Figure 1 Adjusted ORs for the relationship between amyotrophic lateral sclerosis and (A) the intake of fatty acids and cholesterol and (B) flavonoids, lycopene, glutamate, vitamin C and vitamin E. The ORs were adjusted for sex, age (age at onset for patients), level of education (low, middle, high), smoking (never, ever, current), body mass index (underweight, normal, overweight) and total energy intake according to the residual method; SATFA, saturated fatty acids; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Interaction analysis showed that the ORs for vitamin E were further reduced from 0.67 to 0.37 (p = 0.02), and for PUFA from 0.60 to 0.26 (p = 0.005), with a significant interaction term (p = 0.03).
Flavonols, lycopene, vitamin B2 (not shown) and vitamin C did not show any significant association. Analysis of glutamate, calcium and phytoestrogens all yielded adjusted p>0.73.
Daily nutrient intake and clinical features
No salient association between PUFA and vitamin E intake and age at onset or duration of disease was found with multivariate Cox regression analysis. PUFA and duration of disease: hazard ratio (HR) = 0.94, 95% CI = 0.7 to 1.3, p = 0.72. PUFA and age at onset: HR = 0.94, 95% CI = 0.7 to 1.2, p = 0.63. Vitamin E and duration of disease: HR = 1.2, 95% CI = 0.9 to 1.7, p = 0.18. Vitamin E and age at onset: HR = 0.93, 95% CI = 0.7 to 1.2, p = 0.52.
Discussion
We investigated the association between intake of nutrients and the risk of developing ALS, as we hypothesised that daily nutrient intake can modify observed pathological processes in ALS, including oxidative stress, mitochondrial dysfunction, apoptosis, inflammation and glutamate excitotoxicity. This study showed that higher premorbid dietary intake of PUFAs and vitamin E was associated with a 50–60% decreased risk of developing ALS. These associations were independent of possible confounding factors and total energy intake.
The finding that a higher intake of PUFAs appeared to decrease the risk of developing ALS may be in accordance with the results of studies in patients with other primarily neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease.12,28 Omega 3 PUFAs—eicosapentanoic acid, docosahexanoic acid and alpha‐linolenic acid—in particular, have been shown to protect against cardiovascular disease and Alzheimer's disease.29 In contrast, omega 6 fatty acids—linoleic acid—have opposite mechanisms of action.29 Arachidonic acid—derived from linoleic acid and eicosapentanoic acid—compete for the cyclooxygenase enzyme for conversion into prostaglandins. The prostaglandins derived from omega 6 are proinflammatory and those derived from omega 3 are anti‐inflammatory.29 Inflammation and upregulation of cyclooxygenase have both been described in ALS.30 One previous study, however, found an association between a higher intake of PUFAs and a higher risk of developing ALS.4 The fact that the current food‐frequency questionnaire was validated specifically for fat and cholesterol may partially explain this discrepancy.15 Also, because neither study differentiated between omega 3 and omega 6 PUFAs, differences between an American and European study population in consumption of specific foodstuffs that contain predominantly omega 3 (fish, dark green leafy vegetables) or omega 6 (cereals, whole‐grain bread, baked goods, fried foods) PUFAs may also have contributed to these discrepancies.
Another possible mechanism of action of PUFAs is direct neuroprotection through attenuation of glutamate excitotoxicity.31 Omega 3 fatty acids—for example, alpha‐linoleic acid—have been shown to protect neurones from kainate‐induced cell death, probably through ion channels that are also activated by riluzole—the only drug currently effective in ALS.31 Furthermore, a protective effect of PUFA intake may also suggest a link with cardiovascular disease, similar to Alzheimer's disease.32 Chronic hypoperfusion with hypoxia could contribute to mitochondrial dysfunction and motor neurone death in ALS. The recent finding that vascular endothelial growth factor may be implicated in the process of motor neurone degeneration in ALS33 either reaffirms a defect in neurotrophin biology or suggests a role for chronic hypoxia.
The observed protective effect of vitamin E intake and the risk of developing ALS is in contrast with two previous studies that showed a lack of association.4,8 In a subsequent large study, however, regular users of vitamin E supplements had a 40–50% reduced risk of developing ALS.34 Also, the observation that vitamin E delays clinical expression in the mouse model of ALS35 and the known inhibitory effects of vitamin E on lipid peroxidation36 support a role for vitamin E in modifying the risk of developing ALS. 4‐Hydroxynonenal, itself a product of lipid peroxidation in vivo, has been shown to be bound to the glutamate transporter in patients with ALS,37 thus damaging the transporter and contributing to glutamate excitotoxicity. The combined analysis, including the interaction term, indicates that vitamin E and PUFAs increase their separate protective effects. Vitamin E may act directly to reduce the risk of ALS as a known inhibitor of lipid peroxidation, but it could also act indirectly through inhibition of peroxidation of nutritional PUFAs. As a result, a higher level of PUFAs will be available biologically.
As male sex is an established risk factor and late menarche and early menopause seem to occur in women with ALS,38 intake of phytoestrogens was investigated in this study. The intake of phytoestrogens was found to be similar in patients and controls.
Premorbid dietary intake of glutamate was previously shown to be higher in patients with ALS,4 suggesting a possible dietary contribution to glutamate excitotoxicity. This study, however, did not show any sign of premorbid increased glutamate intake. Differences in the food‐frequency questionnaires that were used probably contributed to this discrepancy. Nevertheless, it is unlikely that dietary intake levels of glutamate are sufficient to cause marked changes in levels of glutamate in the brain and spinal cord, because glutamate levels in the central nervous system are tightly regulated at the blood–brain barrier.39,40
The limitations of this study are the possible influence of overmatching of controls, recall bias and the non‐population‐based design. Overmatching, however, leads to false‐negative findings, further emphasising the positive finding of this study. The effect of recall bias will also be small because patients were not informed about our hypotheses regarding vitamin E or PUFAs, and these data were calculated from general dietary questions. Importantly, this case–control study took into account the possible influence of preclinical disease in assessing dietary intake and adjusted for important confounders, including total energy intake, according to the residual method.26 A population‐based case–control study is presently being conducted to generate class I evidence.
Acknowledgements
This study was supported by a grant from ZonMw, The Netherlands Organization for Health Research and Development.
Abbreviations
ALS - amyotrophic lateral sclerosis
BMI - body mass index
PUFA - polyunsaturated fatty acid
Footnotes
Competing interests: None.
The institutional ethical committee of the University Medical Center Utrecht approved the study protocol.
References
- 1.Rowland L P. What's in a name? Amyotrophic lateral sclerosis, motor neuron disease, and allelic heterogeneity. Ann Neurol 199843691–694. [DOI] [PubMed] [Google Scholar]
- 2.Armon C. An evidence‐based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology 200322217–228. [DOI] [PubMed] [Google Scholar]
- 3.Shaw P J. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 2005761046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson L M, Matkin C, Longstreth W T., Jret al Population‐based case‐control study of amyotrophic lateral sclerosis in western Washington State. II. Diet. Am J Epidemiol 2000151164–173. [DOI] [PubMed] [Google Scholar]
- 5.Sienko D G, Davis J P, Taylor J A.et al Amyotrophic lateral sclerosis. A case‐control study following detection of a cluster in a small Wisconsin community. Arch Neurol 19904738–41. [DOI] [PubMed] [Google Scholar]
- 6.Felmus M T, Patten B M, Swanke L. Antecedent events in amyotrophic lateral sclerosis. Neurology 197626167–172. [DOI] [PubMed] [Google Scholar]
- 7.Pierce‐Ruhland R, Patten B M. Repeat study of antecedent events in motor neuron disease. Ann Clin Res 198113102–107. [PubMed] [Google Scholar]
- 8.Longnecker M P, Kamel F, Umbach D M.et al Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology 200019210–216. [DOI] [PubMed] [Google Scholar]
- 9.Bergomi M, Vincelli M, Rovesti S.et al Epidemiology of amyotrophic lateral sclerosis in Italy 1988–1993. Epidemiology 1997835 [Google Scholar]
- 10.Savettieri G, Salemi G, Arcara A.et al A case‐control study of amyotrophic lateral sclerosis. Neuroepidemiology 199110242–245. [DOI] [PubMed] [Google Scholar]
- 11.den Hartog Jager W A, Hanlo P W, Ansink B J.et al Results of a questionnaire in 100 ALS patients and 100 control cases. Clin Neurol Neurosurg 19878937–41. [DOI] [PubMed] [Google Scholar]
- 12.Lau de L M L, Bornebroek M, Witteman J C M.et al Dietary fatty acids and the risk of Parkinson disease. Neurology 2005642040–2045. [DOI] [PubMed] [Google Scholar]
- 13.Brooks B R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994124(Suppl)96–107. [DOI] [PubMed] [Google Scholar]
- 14.Van den Berg‐Vos R M, Visser J, Franssen H.et al Sporadic lower motor neuron disease with adult onset: classification of subtypes. Brain 20031261036–1047. [DOI] [PubMed] [Google Scholar]
- 15.Feunekes G I, Van Staveren W A, De Vries J H.et al Relative and biomarker‐based validity of a food‐frequency questionnaire estimating intake of fats and cholesterol. Am J Clin Nutr 199358489–496. [DOI] [PubMed] [Google Scholar]
- 16.Brussaard J H, Hulshof K F, Kistemaker C.et al Adequacy of the iodine supply in The Netherlands. Eur J Clin Nutr 199751(Suppl 4)S11–S15. [PubMed] [Google Scholar]
- 17.Hulshof K F, Lowik M R, Kistemaker C.et al Comparison of dietary intake data with guidelines: some potential pitfalls (Dutch nutrition surveillance system). J Am Coll Nutr 199312176–185. [DOI] [PubMed] [Google Scholar]
- 18.Hertog M G, Hollman P C, Katan M B.et al Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer 19932021–29. [DOI] [PubMed] [Google Scholar]
- 19.Hertog M G, Feskens E J, Hollman P C.et al Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 19933421007–1011. [DOI] [PubMed] [Google Scholar]
- 20.USDA‐Iowa State University Database http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav.html
- 21.Rhodes J, Titherley A C, Norman J A.et al A survey of the monosodium glutamate content of foods and an estimation of the dietary intake of monosodium glutamate. Food Addit Contam 19918663–672. [DOI] [PubMed] [Google Scholar]
- 22.Loliger J. Function and importance of glutamate for savory foods. J Nutr 2000130915S–20S. [DOI] [PubMed] [Google Scholar]
- 23.Skurray G R, Pucar N L. Glutamic acid content of fresh and processed foods. Food Chem 27 1988177–180.
- 24.Yamaguchi S, Ninomiya K. Umami and food palatability. J Nutr 2000130921–926. [DOI] [PubMed] [Google Scholar]
- 25.Glutamate content of foods http://www.msgfacts.com/chart.html.2000 (accessed 5 Apr 2006)
- 26.Willett W, Stampfer M J. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 198612417–27. [DOI] [PubMed] [Google Scholar]
- 27.Traynor B J, Codd M B, Corr B.et al Incidence and prevalence of ALS in Ireland, 1995–1997: a population‐based study. Neurology 199952504–509. [DOI] [PubMed] [Google Scholar]
- 28.Kalmijn S, Launer L J, Ott A.et al Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 199742776–782. [DOI] [PubMed] [Google Scholar]
- 29.Din J N, Newby D E, Flapan A D. Omega 3 fatty acids and cardiovascular disease‐‐fishing for a natural treatment. BMJ 200432830–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasojima K, Tourtellotte W W, McGeer E G.et al Marked increase in cyclooxygenase‐2 in ALS spinal cord. Neurology 200557952–956. [DOI] [PubMed] [Google Scholar]
- 31.Lauritzen I, Blondeau N, Heurteaux C.et al Polyunsaturated fatty acids are potent neuroprotectors. EMBOJ 2000191784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelhart M J, Geerlings M I, Ruitenberg A.et al Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 20022873223–3229. [DOI] [PubMed] [Google Scholar]
- 33.Lambrechts D, Storkebaum E, Morimoto M.et al VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genetics 200334383–394. [DOI] [PubMed] [Google Scholar]
- 34.Ascherio A, Weisskopf M G, O'Reilly E J.et al Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol 200557104–110. [DOI] [PubMed] [Google Scholar]
- 35.Gurney M E, Cutting F B, Zhai P.et al Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann Neurol 199639147–157. [DOI] [PubMed] [Google Scholar]
- 36.Garrow J S. Fat‐soluble vitamins. In: Garrow JS, James WPT, eds. Human nutrition and dietetics. London: Churchill Livingstone, 2000239–264.
- 37.Pedersen W A, Fu W, Keller J N.et al Protein modification by the lipid peroxidation product 4‐hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol 199844819–824. [DOI] [PubMed] [Google Scholar]
- 38.Chio A, Meineri P, Tribolo A.et al Risk factors in motor neuron disese: a case‐control study. Neuroepidemiology 199110174–184. [DOI] [PubMed] [Google Scholar]
- 39.Colombo J P, Cervantes H, Kokorovic M.et al Effect of different protein diets on the distribution of amino acids in plasma, liver and brain in the rat. Ann Nutr Metab 19923623–33. [DOI] [PubMed] [Google Scholar]
- 40.Fernstrom J D. Dietary amino acids and brain function. J Am Diet Assoc 19949471–77. [DOI] [PubMed] [Google Scholar]