Abstract
Background
In multiple sclerosis (MS), multiple periventricular lesions are commonly the first findings on MRI. However, most of these MS lesions are clinically silent. The brain atrophy rate has shown better correlation to physical disability, but it is not clear how atrophy develops over decades. Corpus callosum forms the roof of the third and lateral ventricles. The corpus callosum area (CCA) in a midsagittal image is age independent in a normal adult population up to the seventh decade; therefore it can be used as a marker for non‐age‐related, pathological brain atrophy.
Objectives
To investigate whether and how CCA decreases in size over time in patients with MS.
Methods
In a clinical observational study, 37 patients with MS with a wide range of disease duration at baseline (1–33 years) were followed. Three different MS courses were represented. The mean of individual MRI follow‐up was 9 years. Multiple sclerosis severity score (MSSS) was also applied to evaluate disability at baseline and after 9 years of follow‐up.
Results
A significant decrease in CCA over 9 years (p<0.001) and a persisting association between CCA and the disability status were found. The atrophy rate was similar ever four decades of MS for all MS courses. The mean annual CCA decrease was 9.25 mm2 (1.8%). Surprisingly, atrophy rate did not correlate with sex, disease duration, age at MS onset or MS course.
Conclusions
Serial evaluations of CCA might be a robust method in monitoring a non‐age‐related decrease in CCA, reflecting progression of irreversible destructive changes in MS.
Multiple sclerosis (MS) is a complex inflammatory disease of the brain and spinal cord,1,2,3 which leads to a well‐documented early irreversible atrophy.4,5,6 The main neuroimaging modality used to monitor MS development is MRI, which can visualise both lesions and atrophy. In follow‐up examinations of patients with MS, the correlation between clinical development and extent of MRI findings is generally poor, which is sometimes referred to as “the clinicoradiological paradox”.7
In contrast with focal MS lesions, atrophy measures of the brain or spinal cord have been regarded as a better predictor of the disability progression in MS.2,5,8,9,10 However, some reports also show non‐significant correlation between disability and atrophy.11,12,13,14,15,16 Focal MS lesions visualised on MRI have a characteristic pattern of oval‐shaped, typically periventricular white matter changes, often located in the corpus callosum. Atrophy of the corpus callosum is common in MS. However, pathological changes in the corpus callosum might develop independently of focal T2‐weighted lesions.17
The corpus callosum, consisting of 2×108 axons in a healthy person, forms the roof of the third and lateral ventricles and has a central role for interhemispheric communication.18 The corpus callosum area (CCA) is normally resistant to age‐related shrinkage between the third and the seventh decades of life.19,20 Atrophy of the corpus callosum correlates to other measures of brain atrophy such as widening of third and lateral ventricles.1 Pelletier et al21 reported a persisting association between CCA and disability, as assessed by the Expanded Disability Status Scale (EDSS) in a 5‐year longitudinal study of patients with relapsing–remitting multiple sclerosis (RRMS). Schreiber et al22 reported CCA in patients with MS to be associated with EDSS. In contrast, Barkhof et al23 reported a lack of correlation between CCA and EDSS. Simon et al1 found a slight correlation between CCA and EDSS at baseline, but on follow‐up there was no significant correlation between the significant CCA decrease and EDSS change.
The corpus callosum atrophy rate has not been reported for different disease durations, sex or types of MS course in longitudinal studies.21 The starting point for prospective, longitudinal MRI studies is often close to the time of diagnosis of MS, focusing on the early years of the disease.
We followed a patient cohort for 9 years. Disease duration at baseline was widespread (range 1–33 years), giving us the possibility of an overview of disease development over four decades. Our first aim was to study the rate at which the callosal atrophy developed. Second, we wanted to study the correlation between the atrophy rate and disability changes. The third aim was to study the association between CCA and disability at baseline and at the end of the study. The fourth aim was to investigate the association of the atrophy rate to sex, MS course (course at the end of study), disease duration and age at onset.
Methods
Subjects
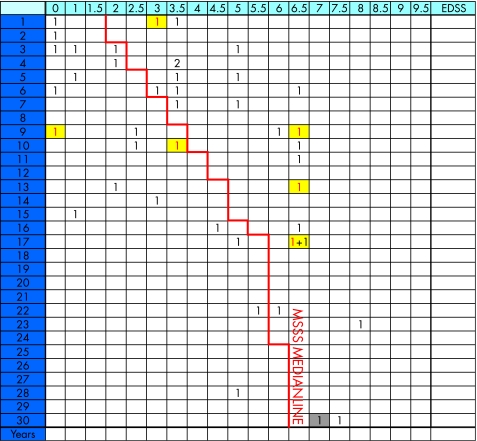
This was a follow‐up study of an original study group of 45 patients with MS, which consisted of consecutive patients selected from the outpatient ward at the Clinic of Neurology, Karolinska University Hospital in Stockholm, Sweden, during 1995–6. The ethics committee at Karolinska Institutet approved the study. All patients gave their informed consent. At the time of inclusion, all patients had MS according to the revised McDonald criteria.24 In all, 24 of the 37 patients who could be tracked had received interferon treatment during the follow‐up period. There was no randomised selection or available information of the compliance for medication. Six patients converted from RRMS to secondary‐progressive multiple sclerosis (SPMS) during follow‐up and were included in the SPMS group for statistical analyses. One of 37 patients was not investigated with sagittal T2‐weighted images at baseline. Six of eight patients lost to follow‐up are described in the multiple sclerosis severity score (MSSS) table (fig 1). They were found to be distributed on both sides of the median MSSS characteristic, indicating a lesser risk of selection bias. Of the eight patients lost to follow‐up, one emigrated, one had postoperative contraindicating metal clips, two died, and the remaining four refused to participate or had no contact with the MS clinic. Six patients improved in disability, with a mean EDSS score of 0.8 (range 0.25–1.25) during the 9‐year follow‐up. Four of these patients had fluctuating EDSS scores between the first and second measurements in 1995–6. There was no second EDSS score for two patients in 1996. Therefore, we calculated a mean EDSS value of both the first and second EDSS scores in 1995 and 1996.
Figure 1 The Roxburgh global Multiple Sclerosis Severity Score (MSSS) table14 used to illustrate patient baseline Expanded Disability Status Scale (EDSS) status (1995–6). In all, 43 of a total of 45 patients are assigned MSSS scores based on EDSS and duration. The left side of the median represents slower progression and the right side faster progression. Patients lost to follow‐up are marked with red numbers on a yellow background. One patient with 33 years disease duration at baseline (grey background) was placed as 30 years duration. For two patients, data concerning onset of multiple sclerosis and follow‐up were lacking and could not be included.
Both clinical and radiological follow‐up examinations were performed at the Karolinska University Hospital in 1995–6 and 2003–5. Clinical disability was evaluated by neurologists specialised in MS using EDSS,25 and additionally adjusted for disease duration and expressed as MSSS.26
Magnetic resonance imaging protocol
The MRI examinations were performed as sagittal T2‐weighted MR images at baseline (1995–6) and at the end of the study (2003–5) using 1.5 T scanners with a standard head coil (1995–6 GE Signa and 2003–5 Siemens Magnetom Vision). Field of view (25 cm) and matrix (256×256) were the same for both examinations.
In 1995–6, scans were performed using fast spin echo, TR/TE/FA/NEX 4000/76/90/1. Slice thickness was 5 mm with no gaps.
In 2003–5, scans were performed with turbo spin echo, TR/TE/FA/NEX 3500/96/180/2, with 4 mm thick slices and distance 0.4 mm.
Image processing
The MR data were analysed by a radiologist blinded to the clinical data, using a PACS workstation (Sectra, Sweden). Midsagittal callosum area and total intracranial area were measured on T2‐weighted sagittal images in the midline. For orientation, local anatomy including cerebrospinal fluid‐filled interhemispheric fissure, anterior commissure, posterior commissure and the vein of Galen were used as landmarks and reference points. Midline internal skull surface area (MISS) was delineated as the bony margins of skull and reflected the total intracranial volume. Intracranial volume (three‐dimensional) is age independent in adulthood19—that is, (two‐dimensional) MISS is also constant over time.19
Corpus callosum measurements were repeated three times, and a mean value was calculated. MISS was measured once for each MRI scanning. To ensure comparability of serial callosal measurements, a normalised ratio was applied by dividing the mean CCA by MISS. This CCA/MISS ratio19 was used for internal calibration control because of the long (9 years) interval between the two MRI examinations, the use of different scanners (at baseline and at the end) and slightly different imaging parameters. The normalised ratio has been based on the fact that skull volume is known to be stable in adulthood.19
Statistical analysis
The gradient between longitudinal measurements of CCA during the years 1995–6 and 2003–5 was calculated by performing simple linear regression for every patient with MS.27 Multivariable linear regression models were then fitted to investigate the influence of type of MS course, disease duration, interferon treatment, age at MS onset and sex on the new outcome—that is, the decrease in CCA. Since the baseline level of CCA might have an effect on the CCA decrease, the CCA at entry was included as a covariate in the model.
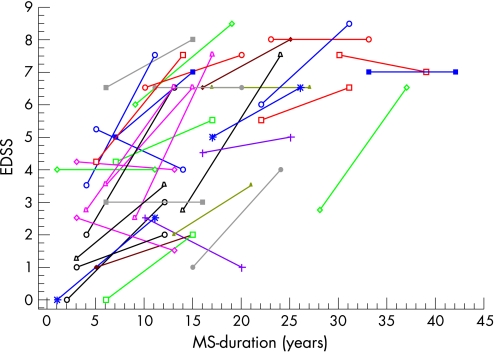
The EDSS is an ordinal score variable and thus the change in EDSS was classified into two categories, increased and decreased/unchanged. Due to the sparse number of decreased and unchanged cases, exact logistic regression was performed. The effect of MS course, duration at baseline, age at MS onset, sex and CCA progression on the probability of increased EDSS was evaluated. Exact logistic regression was performed in the same manner for individual MSSS. Spearman's rank correlation was used to evaluate the relationship between EDSS/MSSS versus CCA at baseline and at end, using the original ordinal values of EDSS/MSSS. The results of the exact logistic regression analyses are presented as odds ratios. Figure 2 illustrates the individual progress of EDSS during follow‐up.
Figure 2 Individual Expanded Disability Status Scale (EDSS) development over 9 years of follow‐up for 37 patients. 25 patients manifested an increase in EDSS, six were stable and six patients showed a decrease in EDSS. MS, multiple sclerosis.
A backward selection procedure was carried out to investigate which of the independent variables could explain most of the variation in EDSS, CCA and MSSS. An exclusion criterion of p>0.05 was used at every step of the process to determine which variables should be excluded. Duration and baseline measurement covariates were included in all models regardless of any p value.
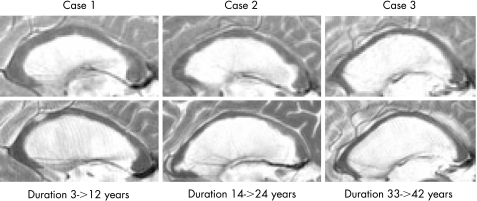
Cook's distance and residual plots were used to detect any observations that had a large influence on the regression results. The mean annual atrophy rate (in relative values) was not analysed, but is referred to in the Results section (in absolute values). This ratio was calculated by taking the ratio between the measurements during 2003–5 and during 1995–6. To account for the patients' follow‐up time, the ratio was divided by time between the beginning and the end of the study (fig 3). An intraclass correlation was calculated to assess the agreement between CCA‐normalised ratio and CCA‐non‐normalised area measurements (mm2).28
Figure 3 MRI T2 weighted sagittal images showing thinning of the corpus callosum during follow‐up in three patients at baseline (above) and at the end of the study (below).
Statistical analyses were performed using SAS software V.9.1.3 and LogXact V.7.0. Graphical presentations and intraclass correlations were performed using the Statistica V.7.0 software.
Results
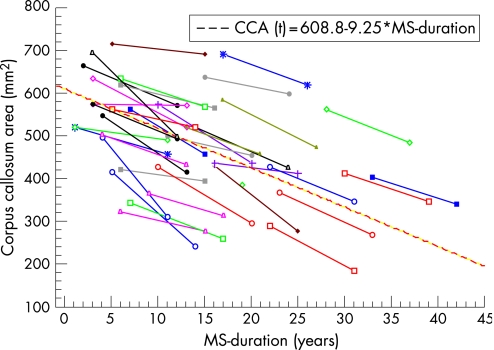
The mean annual callosal area decrease was 9.25 mm2 (p<0.001; 95% CI −11.15 to −7.35), corresponding to an annual decrease of 1.8% (95% CI 1.39 to 2.22), surprisingly constant over four decades of the disease (table 1, fig 4). Comparison between normalised and non‐normalised CCA measurements showed an intraclass correlation of 0.975. This strongly assures comparability of measurements between MRI scans at baseline and at the end of the study. Strikingly, the atrophy rate was not associated with sex, disease duration at baseline, age at MS onset, interferon treatment, MS course or baseline CCA. The Roxburgh global MSSS model showed the physical disability status at baseline (fig 1). Follow‐up EDSS data varied (fig 2); the majority of patients worsened in their disability (n = 25), while some of them were stable (n = 6) or improved (n = 6). The exact logistic regression showed no significant relationship between the atrophy rate and the progression of EDSS (table 2) or MSSS (table 3). Spearman's rank correlation between CCA and EDSS/MSSS at baseline was 0.57 (p<0.05)/0.52 (p<0.05) and at the end 0.58 (p<0.05)/0.52 (p<0.05), showing a persistent moderate to strong association between CCA and EDSS/MSSS over the follow‐up time.
Table 1 Characteristics of the study group at the end of the study.
| Age at baseline* (years) | Age at MS onset* (years) | CCA* (mm2) | Individual EDSS Prob (increase)† | Individual MSSS Prob (increase)† | MRI follow‐up* (years) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 48.6/9.53/26 | 29.9/8.13/26 | −9.21/7.60/25 | 0.65/17/26 | 0.61/6/23 | 9.31/0.83/26 |
| Male | 56.5/10.40/11 | 32.9/8.62/11 | −9.26/4.7/11 | 0.72/8/11 | 0.75/14/8 | 9.09/0.68/11 |
| Interferon | ||||||
| Treatment | 50.7/11.02/24 | 31.3/8.61/24 | −8.44/5.72/23 | 0.79/19/24 | 0.81/17/21 | 9.13/0.80/24 |
| Non‐treatment | 52.1/9.48/13 | 30/7.92/13 | −9.71/5.65/13 | 0.46/6/13 | 0.3/3/10 | 9.46/0.52/13 |
| MS course | ||||||
| RRMS | 47.9/7.54/16 | 31.3/9.21/16 | −8.52/5.84/16 | 0.63/10/16 | 0.60/9/15 | 9.43/0.63/16 |
| SPMS | 52.2/11.81/17 | 29.1/7.18/17 | −10.90/6.42/16 | 0.71/12/17 | 0.62/8/13 | 9.11/0.88/17 |
| PPMS | 60/9.97/4 | 36.3/8.30/4 | −5.61/2.34/4 | 0.75/3/4 | 1.00/3/3 | 9.0/0/4 |
| Overall | 51.2/8.29/37 | 30.8/8.28/37 | −9.25/5.62/36 | 0.67/25/37 | 0.65/20/31 | 9.24/0.72/37 |
CCA, corpus callosum area; EDSS, expanded disability status scale; MS, multiple sclerosis; MSSS, MS severity score; RRMS, relapsing–remitting MS; SPMS, secondary‐progressive MS; PPMS, primary‐progressive MS.
MSSS scale ends at 30 years disease duration—that is, we could monitor only 31 of 37 patients at the end.
*Mean gradient/SD/number of patients.
†Probability/number of patients with increase/number of patients.
Figure 4 Individual evolution of corpus callosum area (CCA), measured at baseline and at the end of the study. According to the regression line, the CCA decreases on average by 9.25 mm2 per year (1.80%).
Table 2 ORs, 95% CIs and p values for the analysis of individual EDSS.
| Model term | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Duration | 0.97 | 0.89 | 1.05 | >0.5 |
| CCA decrease (diff)* | 1 | 0.98 | 1.01 | >0.5 |
CCA, corpus callosum area; EDSS, Expanded Disability Status Scale.
*CCA corrected for participants' time in study.
Table 3 Odds ratios, 95% confidence intervals and p values for the analysis of individual MSSS.
| Model term | OR | 95% CI | p Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Duration | 0.95 | 0.8 | 1.14 | >0.5 |
| CCA decrease (diff)* | 1 | 0.99 | 1.02 | >0.5 |
CCA, corpus callosum area; MSSS, multiple sclerosis severity score.
*CCA corrected for participants' time in study.
Discussion
To our knowledge, this is the longest follow‐up study investigating the atrophy rate of the corpus callosum in MS. The study showed a progressive atrophy over four decades of disease duration independent of both MS course and physical disability change. This may be interpreted as a support for the evolving concept of MS as a steadily progressing neurodegenerative disease in which signs of inflammation such as relapses or focal lesions are less relevant in longitudinal follow‐up observations.
Focal lesions visible on MRI are important diagnostic features of MS, but they correlate poorly with clinical progression. Neuroaxonal atrophy is an irreversible process, whereas focal lesions fluctuate over time. Thus, changes in brain and spinal cord volumes have become increasingly strong candidates to correlate better with the long‐term development of disability in MS.
There are several pathological and physiological variables that may affect brain volume evaluations in MS. Variables causing an increase in brain volume are oedema, inflammation, gliosis (tissue bulk) and remyelination. Variables causing a decrease in brain volume are axonal loss, resolution of both inflammation and oedema, gliosis (retraction scarring), demyelination, dehydration, normal ageing and influence of anti‐inflammatory treatment.29 This complexity is likely to add to the uncertainty related to the atrophy measurements in MS. For instance, it is still not known at what rate atrophy develops over longer periods of time. There might be an atrophy threshold for disability progression, and critical location of atrophy might be important for disability worsening. Differences in focal atrophy rates between different MS courses are still inadequately understood.
Some studies have reported differences between MS courses with regard to the rate of brain atrophy. Longitudinal studies of RRMS have shown annual atrophy rates between 0.5% and −0.8%.30,31,32,33 In SPMS, atrophy rates between 1.3% and 1.4% have been reported,30,33,34 whereas patients with PPMS had annual atrophy rates of 1% and 1.3% in two longitudinal studies.35,36 Rudick et al12 reported a correlation between whole brain atrophy and callosal atrophy.
Atrophy of the corpus callosum has been suggested to reflect periventricular white matter changes and also Wallerian degeneration.37 Pelletier et al21 reported that callosal atrophy can be detected in early stages of MS and with mild disability in RRMS. These findings suggest that callosal atrophy may serve as an early morphological marker of corpus callosum involvement. Simon et al1 reported similar results in his 2‐year longitudinal study of brain atrophy. They found significant callosal atrophy as well as an increase in ventricular volume after 1 year of MRI follow‐up, and an annual decrease of corpus callosum by 4.9% for patients with RRMS using 5 mm slices.1 Furthermore, Simon et al1 reported a small, but significant correlation between the CCA and EDSS at baseline, but no further significant correlation between CCA decrease and EDSS change at the end of the study. Their results are in agreement with our results. Furthermore, Pelletier et al21 reported a persisting association between CCA and EDSS in a 5‐year longitudinal study of patients with RRMS when using 5 mm MRI slices. Their results are in accordance with our results. They reported an association of impaired auditory, motor and sensory interhemispheric transfer as well as callosal atrophy.21 Schreiber et al reported CCA in patients with MS to be associated with EDSS.22 However, Barkhof et al23 reported an insignificant correlation of CCA to both EDSS and duration of MS. Our data did not show a significant correlation with disability change during the follow‐up time.
Longitudinal studies of atrophy in MS are uncommon.1,12,35 Fisher et al,5 in an 8‐year follow‐up study, reported a significant progression of whole brain atrophy with correlation to EDSS change in RRMS. Kalkers et al13 reported that brain atrophy is independent of MS course. Furthermore, they found no correlation between the baseline EDSS or the annualised ΔEDSS score and annualised markers of brain atrophy.13 Similarly, we did not find any significant differences between the three MS courses and atrophy rate in our study. In a recent longitudinal study, Pagani et al38 showed that brain atrophy develops involving different structures in different MS courses, corpus callosum atrophy being typical for RRMS.
We found a mean CCA at baseline of 5.05 cm2, which is comparable to the area of 5.3 cm2 in the MS study by Barkhof et al23 and 5.5 cm2 in that by Simon et al.1 CCA of our patients was significantly smaller than normal CCA in a healthy population.19,20 Sullivan et al19 and Mitchell et al20 found no age effect for corpus callosum size in a normal population. Mitchell et al reported a mean normal CCA of 6.27 cm2 in a healthy population with an age range of 14–68 years.20 These results are similar to the extrapolated area at MS onset pointed out by the regression line shown in fig 4. This strongly suggests that our patients had a mean CCA equal to that in a normal healthy population before symptom onset.19,20 Neither disease duration at baseline nor CCA at baseline predicted the atrophy rate (table 4).
Table 4 Analysis of variance for results on decrease in corpus callosum area.
| Model term | Estimate | SE (estimate) | 95% CI | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Disease duration (at baseline) | 0.05 | 0.12 | −0.2 | 0.29 | >0.5 |
| CCA baseline | 0 | 0.01 | −0.02 | 0.02 | >0.5 |
CCA, corpus callosum area.
It is necessary to consider some limitations of our study. First, EDSS is known to put emphasis on motor function and largely lacks input of cognitive assessment—that is, it may assess spinal processes rather than cerebral ones.39 Another problem in using EDSS is its lack of linearity. The MSSS has been developed to classify patients or groups of patients into severity groups, taking into account duration of the disease. We used the MSSS to assess relative severity at baseline and at 9 years of follow‐up.
Technically there were some differences that could potentially affect the evaluations. Slice thickness used at baseline was 5 mm, whereas that used at follow‐up was 4 mm. Measurements could also be biased for technical reasons such as slice location and partial volume effects. Intracranial (three‐dimensional) volume (the bony margins of skull internal surface) is known to be constant in adulthood19—that is, the inner (two‐dimensional) skull surface area measurement (MISS) is constant.19 By using MISS, we could normalise our corpus callosum measurements19 to ensure that our results are not biased by the use of different MRI scanners. The strikingly high intra‐class correlation between normalised and non‐normalised measurements suggested a high degree of comparability.
Conclusion
We found a highly significant, non‐age‐related progression of the corpus callosum atrophy in a 9‐year follow‐up study of patients with MS representing four decades of disease development, with a persisting association between CCA and the disability status. We did not find significant variation in atrophy rate between the three MS courses. Serial evaluations of CCA might be a robust method in monitoring a non‐age‐related decrease in CCA, reflecting progression of irreversible changes in MS. These type of evaluations are simple to apply and therefore might be useful for radiological monitoring of follow‐up studies and trials.
Abbreviations
CCA - corpus callosum area
EDSS - Expanded Disability Status Scale
MISS - midline internal skull surface area
MS - multiple sclerosis
MSSS - multiple sclerosis severity score
RRMS - relapsing–remitting multiple sclerosis
SPMS - secondary‐progressive multiple sclerosis
Footnotes
Competing interests: None declared.
References
- 1.Simon J H, Jacobs L D, Campion M K.et al A longitudinal study of brain atrophy in relapsing multiple sclerosis. Neurology 199953139–148. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Edwards S, Gong Q.et al Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry 199966323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermel R A, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 20065158–170. [DOI] [PubMed] [Google Scholar]
- 4.Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging 20041427–35. [DOI] [PubMed] [Google Scholar]
- 5.Fisher E, Rudick R A, Simon J H.et al Eight‐year follow‐up study of brain atrophy in patients with MS. Neurology 2002591412–1420. [DOI] [PubMed] [Google Scholar]
- 6.Brex P A, Jenkins R, Fox N C.et al Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology 2000541689–1691. [DOI] [PubMed] [Google Scholar]
- 7.Barkhof F. The clinico‐radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 200215239–245. [DOI] [PubMed] [Google Scholar]
- 8.Bakshi R, Benedict R H, Bermel R A.et al Regional brain atrophy is associated with physical disability in multiple sclerosis: semiquantitative magnetic resonance imaging and relationship to clinical findings. J Neuroimaging 200111129–136. [DOI] [PubMed] [Google Scholar]
- 9.Ingle G T, Stevenson V L, Miller D H.et al Primary progressive multiple sclerosis: a 5‐year clinical and MR study. Brain 20031252528–2536. [DOI] [PubMed] [Google Scholar]
- 10.Bakshi R, Dandamudi V S R, Neema M.et al Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 20051530–45. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Grossman R I, Udupa J K.et al Brain atrophy in relapsing‐remitting multiple sclerosis and secondary progressive multiple sclerosis: longitudinal quantitative analysis. Radiology 2000214665–670. [DOI] [PubMed] [Google Scholar]
- 12.Rudick R A, Fisher E, Lee J C.et al Brain atrophy in relapsing multiple sclerosis: relationship to relapses, EDSS, and treatment with interferon beta‐1a. Mult Scler 20006365–372. [DOI] [PubMed] [Google Scholar]
- 13.Kalkers N F, Amezine N, Bot J C J.et al Longitudinal brain volume measurement in multiple sclerosis. Arch Neurol 2002591572–1576. [DOI] [PubMed] [Google Scholar]
- 14.Fox N C, Jenkins R, Leary S M.et al Progressive cerebral atrophy in MS: a serial study using registered, volumetric MRI. Neurology 200054807–812. [DOI] [PubMed] [Google Scholar]
- 15.Rovaris M, Comi G, Rocca M A.et al Short‐term brain volume change in relapsing‐remitting multiple sclerosis: effect of glatiramer acetate and implications. Brain 20011241803–1812. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson V L, Smith S M, Matthews P M.et al Monitoring disease activity and progression in primary progressive multiple sclerosis using MRI: sub‐voxel registration to identify lesion changes and detect cerebral atrophy. J Neurol 20022171–177. [DOI] [PubMed] [Google Scholar]
- 17.Coombs B D, Best A, Brown M S.et al Multiple sclerosis pathology in the normal and abnormal appearing white matter of corpus callosum by diffusion tensor imaging. Mult Scler 200410392–397. [DOI] [PubMed] [Google Scholar]
- 18.Aboitiz F, Scheibel A B, Fisher R S.et al Fiber composition of the human corpus callosum. Brain Res 1992598143–153. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan E V, Rosenbloom M J, Desmond J E.et al Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging 200122603–611. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell T N, Free S L, Merschhemke M.et al Reliable callosal measurement: population normative data confirm sex‐related differences. Am J Neuroradiol 200324410–418. [PMC free article] [PubMed] [Google Scholar]
- 21.Pelletier J, Suchet L, Witjas T.et al A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing‐remitting multiple sclerosis. Arch Neurol 200158105–111. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber K, Sorensen P S, Koch‐Henriksen N.et al Correlations of brain MRI parameters to disability in multiple sclerosis. Acta Neurol Scand 200110424–30. [DOI] [PubMed] [Google Scholar]
- 23.Barkhof F J, Elton M, Lindeboom J.et al Functional correlates of callosal atrophy in relapsing‐remitting multiple sclerosis patients: a preliminary MRI study. J Neurol 1998245153–158. [DOI] [PubMed] [Google Scholar]
- 24.Polman C H, Reingold S C, Edan G.et al Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 200558840–846. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzke J F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983331444–1452. [DOI] [PubMed] [Google Scholar]
- 26.Roxburgh R H, Seaman S R, Masterman T.et al Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurology 2005641144–1151. [DOI] [PubMed] [Google Scholar]
- 27.Davis C S.Statistical methods for the analysis of repeated measurements. New York: Springer‐Verlag, 21–8,
- 28.Fleiss J L.Design and analysis of clinical experiments. New Ed. New York: Wiley‐Interscience, 199917–28.
- 29.Anderson V M, Fox N C, Miller D H. Magnetic resonance imaging measures of brain atrophy in multiple sclerosis. J Magn Res Imaging 200623605–618. [DOI] [PubMed] [Google Scholar]
- 30.Bermel R A, Sharma J, Tjoa C W.et al A semiautomated measure of whole‐brain atrophy in multiple sclerosis. J Neurol Sci 200320857–65. [DOI] [PubMed] [Google Scholar]
- 31.Chard D T, Griffin C M, Parker G J.et al Brain atrophy in clinically early relapsing‐remitting multiple sclerosis. Brain 2002125327–337. [DOI] [PubMed] [Google Scholar]
- 32.De Stefano N, Matthews P M, Filippi M.et al Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 200371157–1162. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Blumhardt L D, Constantinescu C S. The relationship of brain and cervical cord volume to disability in clinical subtypes of multiple sclerosis: a three‐dimensional MRI study. Acta Neurol Scand 20036401–406. [DOI] [PubMed] [Google Scholar]
- 34.Turner B, Ramli N, Blumhardt L D.et al Ventricular enlargement in multiple sclerosis: a comparison of three‐dimensional and linear MRI estimates. Neuroradiology 20018608–614. [DOI] [PubMed] [Google Scholar]
- 35.Ingle G T, Stevenson V L, Miller D H.et al Two‐year follow‐up study of primary and transitional progressive multiple sclerosis. Mult Scler 20022108–114. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson V L, Miller D H, Leary S M.et al One year follow up study of primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 20006713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon J H, Kinkel R P, Jacobs L.et al A Wallerian degeneration pattern in patients at risk for MS. Neurology 2000541155–1160. [DOI] [PubMed] [Google Scholar]
- 38.Pagani E, Rocca M A, Gallo A.et al Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. Am J Neuroradiol 200526341–346. [PMC free article] [PubMed] [Google Scholar]
- 39.Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 20001231027–1040. [DOI] [PubMed] [Google Scholar]