Abstract
Hyaluronan (HA) is a large glycosaminoglycan that is not only a structural component of extracellular matrices, but also interacts with cell surface receptors to promote cell proliferation, migration, and intracellular signaling. HA is a major component of the extracellular matrix of the distal subapical mesenchymal cells of the developing limb bud that are undergoing proliferation, directed migration, and patterning in response to the apical ectodermal ridge (AER), and has the functional potential to be involved in these processes. Here we show that the HA synthase Has2 is abundantly expressed by the distal subridge mesodermal cells of the chick limb bud and also by the AER itself. Has2 expression and HA production are downregulated in the proximal central core of the limb bud during the formation of the precartilage condensations of the skeletal elements, suggesting downregulation of HA may be necessary for the close juxtaposition of cells and the resulting cell-cell interactions that trigger cartilage differentiation during condensation. Overexpression of Has2 in the mesoderm of the chick limb bud in vivo results in the formation of shortened and severely malformed limbs that lack one or more skeletal elements. Skeletal elements that do form in limbs overexpressing Has2 are reduced in length, exhibit abnormal morphology, and are positioned inappropriately. We also demonstrate that sustained HA production in micromass cultures of limb mesenchymal cells inhibits formation of precartilage condensations and subsequent chondrogenesis, indicating that downregulation of HA is indeed necessary for formation of the precartilage condensations that trigger cartilage differentiation. Taken together these results suggest involvement of HA in various aspects of limb morphogenesis.
Keywords: Hyaluronan, Limb development, Cartilage differentiation, Apical ectodermal ridge
Introduction
Hyaluronan (HA) is a linear glycosaminoglycan composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine that is a structural component of the extracellular matrix of a variety of tissues. It is a huge molecule with molecular weight ranging from 103 to 104 kDa and an extended length of 2–25 μm (see Toole, 1997 see Toole, 2000a,b for reviews). HA occupies an extensive molecular domain in dilute solution, and has a pronounced hydration capacity (Toole, 1997, 2000a,b). At higher concentrations individual extended HA molecules form an entangled continuous hydrated network. Although several of the functions of HA may be attributable to its physicochemical properties, HA is not simply a passive structural and space-filling component of extracellular matrices. Rather, HA directly interacts with cells and influences their behavior. HA concomitantly promotes the proliferation and migration of cells in a number of model systems (Evanko et al., 1999; Itano et al., 2002; Ward et al., 2003; Rilla et al., 2002) and during embryogenesis (Gakunga et al. 1997; Camenisch et al., 2000; Bakkers et al., 2004; Ori et al., 2005). Moreover, interaction of HA with its cell surface receptors including CD44 directly activates intracellular signaling, and can have instructive effects on cell behavior (Turley et al., 2002). HA is an important modulator of the EGFR/ErbB (Camenisch et al., 2002; Tsatas et al., 2002; Bourguignon et al., 1997; Sherman et al., 2000; Ghatak et al., 2005; Misra et al., 2006), TGF-β (Bourguignon et al., 2002), and BMP (Peterson et al., 2004) signaling networks, and acts in concert with these signaling molecules to regulate a variety of cellular processes.
The outgrowth and patterning of the developing limb is regulated by reciprocal interactions between the apical ectodermal ridge (AER), a thickened cap of ectoderm along the distal periphery of the limb bud, and the underlying distal subridge mesoderm (Saunders, 1948). The distal subridge mesenchymal cells of the developing limb bud, which are undergoing proliferation, directed migration, and patterning in response to the AER and other signaling centers such as the zone of polarizing activity (ZPA) produce high amounts of HA which forms an expansive hydrated extracellular matrix between the cells (Toole, 1972; Kosher et al., 1981; Singley and Solursh, 1981; Knudson and Toole, 1985). HA is also synthesized and secreted by the AER itself (Kosher and Savage, 1981). Thus the cell and tissue interactions controlling the outgrowth and patterning of the limb occur in an environment rich in extracellular and pericellular HA.
One of the functions of the AER is to maintain the subridge mesenchymal cells in a labile undifferentiated condition (Kosher et al., 1979). When the mesenchymal cells in the central core of the limb bud become located outside of the range of AER signaling, they initiate cartilage differentiation, the onset of which is characterized by a transient cellular condensation process in which the cells become closely juxtaposed to one another. During this condensation intimate cell-cell interactions occur which are necessary to trigger chondrogenic differentiation (see Kosher, 1983; Solursh, 1983 for reviews). During the initial formation of precartilage condensations, HA production is strikingly downregulated and the hydrated HA-rich extracellular matrix and pericellular HA surface coats are removed (Kosher et al., 1981; Knudson and Toole, 1985) as a result of the receptor-mediated endocytosis of HA and its intracellular degradation by hyaluronidase, in particular Hyal1 (Kulyk and Kosher, 1987; Nicoll et al., 2002). On the basis of these observations it has been suggested that the HA-rich extracellular matrix elaborated by subridge mesenchymal cells may mediate the antidifferentiative effect of the AER by preventing the intimate cell-cell interactions necessary to trigger chondrogenesis (Kosher et al., 1981). Furthermore, the downregulation of HA production and the removal of extracellular HA that occurs when cells in the central core of the limb bud become located outside of the range of AER signaling may be necessary for the close juxtaposition of cells and the resulting cell-cell interactions that trigger chondrogenesis during the formation of precartilage condensations (Kosher et al., 1981).
HA is synthesized in a unique fashion. It is made at the inner side of the plasma membrane rather than in the Golgi apparatus and is extruded to the outside of the cell during polymerization (Weigel et al., 1997). HA is synthesized by three HA synthase isoforms called Has1, Has2, and Has3 (Weigel et al., 1997; Spicer and McDonald, 1998). Has2 is the major source of HA during the period of early organogenesis in the mouse embryo when the early outgrowth and patterning of the limb bud is occurring (Spicer and McDonald, 1998; Camenisch et al., 2000; Tien and Spicer, 2005).
In the present study we demonstrate that Has2 is indeed abundantly expressed by the distal subridge mesodermal cells of the developing chick limb bud that are undergoing outgrowth and patterning in response to the AER and other signaling centers, and is also expressed by the AER itself. Furthermore, Has2 expression is downregulated in the proximal central core of the limb bud during the formation of precartilage condensations. Misexpression of Has2 from the onset of limb development in vivo results in shortened and severely malformed limbs that lack one or more skeletal elements and/or possess skeletal elements that exhibit abnormal morphology or positioning. Finally, we demonstrate that sustained production of HA in micromass cultures of limb mesenchymal cells impairs the formation of precartilage condensations and subsequent chondrogenesis, consistent with the suggestion that downregulation of HA is necessary for the formation of the precartilage condensations that trigger cartilage differentiation.
Materials and methods
Preparation of Has2 adenoviral and retroviral expression vectors and in vivo infection protocol
A recombinant adenoviral expression construct containing a cDNA encompassing the full coding sequence of chicken Has2 (GenBank accession number AF106940) was prepared as previously described (Ward et al., 2003; Zoltan-Jones et al., 2003; Ghatak et al., 2005). Briefly, the cDNA was cloned into the pACCM-V.pLpA shuttle vector, co-transfected into 293 cells with the pJM17 adenovirus, and plaques resulting from successful homologous recombination were chosen, amplified, and purified using cesium chloride gradient centrifugation (Becker et al., 1994; Ward et al., 2003; Zoltan-Jones et al., 2003; Ghatak et al., 2005). The titer of the Has2 adenovirus was 1011 pfu/ml. The Has2 adenoviral mediated synthesis of HA by cells infected with the Has2 adenovirus was confirmed by visualization of HA-dependent pericellular coats using a particle dye exclusion assay (Zoltan-Jones et al., 2003) and by a competitive enzyme-linked immunosorbant assay-like method using biotinylated HA-binding protein (HABP) (Seikagaku America, Inc.) as a probe (Gordon et al., 2003; Zoltan-Jones et al., 2003).
The cDNA containing the full coding sequence of chicken Has2 was also cloned into the avian replication competent retroviral expression vector RCASBP(A), and concentrated Has2 retrovirus (109 cfu/ml) was prepared using standard established protocols as previously described (Ferrari et al., 1998; Ferrari and Kosher, 2002; Fisher et al., 2006). The production of HA by the Has2 RCAS expression vector was confirmed by biotinylated HABP staining of infected chick limb bud mesenchymal cells as described below.
Concentrated Has2 adenovirus or Has2 RCAS retrovirus were microinjected as previously described (Ferrari et al., 1998; Ferrari and Kosher, 2002) into the mesoderm of the right wing buds of stage 18 (Hamburger and Hamilton, 1951) chick embryos or into the prospective limb-forming regions of stage 10 embryos. At various times after microinjection the skeletons of the embryos were stained with Alcian blue and Alizarin Red as previously described (Ferrari and Kosher, 2002) to visualize cartilage and mineralized bone, respectively.
Preparation and infection of micromass cultures
Micromass cultures of mesenchymal cells from stage 22–24 embryonic chick wing buds were established as previously described (Gay and Kosher, 1984) at densities of 2.5, 3.4, and 5 × 104 cells/10 μl of F12 medium containing 10% fetal bovine serum, 1% L-glutamine, and antibiotics. For retroviral infection of the cultures, 1 μl of concentrated Has2 RCAS retrovirus or control RCAS retrovirus lacking the Has2 cDNA insert was added per 10 μl of cell suspensions containing 2.5, 3.4, or 5 × 106 cells/ml, after which 10 μl drops of the suspensions were dispensed onto the surface of 35 mm Nunclon tissues cultures dishes. The retrovirus-containing micromass cultures were incubated for 3 h at 37° C in a humidified CO2 incubator to allow for cell attachment, after which the cultures were supplied with 2.5 ml of medium. Thus, the micromass cultures were exposed to concentrated Has2 or control retrovirus for 3 h during the initial plating and attachment of the cells. The morphology of the cultures was monitored daily by phase contrast microscopy.
Analytical procedures
The accumulation of cartilage matrix by micromass cultures was monitored histochemically by staining with 1% Alcian blue, pH 1.0 as previously described (Gay and Kosher, 1984). The area of cultures occupied by Alcian blue staining cartilage matrix was quantified as previously described (Fisher et al., 2006) using the automated Threshold and Analyze Particles commands in ImageJ image analysis program (version 1.32j) on images acquired under identical exposure and lighting conditions.
The distribution of HA in RCAS control and Has2 RCAS infected micromass cultures was determined by histochemical staining with a biotinylated HA binding protein (HABP) from the HA-binding region of aggrecan which binds strongly and specifically to HA in cells and tissue sections (Knudson and Toole, 1985; Asari et al., 1992; Yu et al., 1996; Shibata et al., 2003; Underhill and Zhang, 2000; Toole et al., 2001). Briefly, cultures that had been fixed in 4% paraformaldehyde were blocked by sequential treatment with avidin, biotin, and 0.1% BSA solutions (Avidin/Biotin Blocking Kit, Vector), incubated with 2 μg/ml of biotinylated HABP (Seikagaku America, Inc) overnight at 4° C, washed with PBT, treated with an avidin-biotin HRP complex (Vectastain ABC kit), washed with PBT, and developed with diaminobenzidene/H2O2 (Vector). No HABP staining was detectable in cultures pretreated for 2 h with 200 TRU/ml of Streptomyces hyalurolyticus hyaluronidase (Seikagaku America, Inc) which specifically degrades HA.
In situ hybridization on serially sectioned limb buds that had been fixed in Bouin’s solution was performed as previously described (Coelho et al., 1991) using a 33P-labeled 517 bp chicken Has2 cDNA probe extending from nucleotides 1490 to 2007 at the 3′ end of the cDNA. The probe was prepared by digesting a pCI-neo vector containing the full coding sequence of chicken Has2 (GenBank accession number AF106940) with XcmI.
Results
Expression of Has2 during early chick limb morphogenesis
Has2 is the major HA synthase that is expressed during the period of early organogenesis in the mouse embryo when the early outgrowth and patterning of the limb bud is occurring (Spicer and McDonald, 1998; Camenisch et al., 2000; Tien and Spicer, 2005). Accordingly, we have examined the temporal and spatial pattern of Has2 expression by in situ hybridization during the early stages of development of the chick limb bud. Has2 is expressed in the posterior subridge mesoderm as early as stage 17/18, which is shortly after formation of the limb bud (Fig. 1A). During early limb morphogenesis from stages 20 (Fig. 1B) through stage 25 (Fig. 1C), Has2 is abundantly expressed by the distal posterior subridge mesenchymal cells of the limb bud that are undergoing outgrowth and patterning in response to the AER and other signaling centers such as the ZPA (Fig. 1C). The distal subridge mesodermal cells that are abundantly expressing Has2 are characterized by a high rate of HA synthesis and are surrounded by an HA-rich extracellular matrix (Kosher et al., 1981; Singley and Solursh, 1981; Knudson and Toole, 1985). In contrast to the distal subridge mesoderm, little or no Has2 expression is detectable in the proximal central core of the limb bud where precartilage condensations are forming (Fig. 1C). The downregulation of Has2 expression in the chondrogenic proximal central core of the limb bud correlates with the downregulation of HA production that occurs during precartilage condensation formation (Kosher et al., 1981; Knudson and Toole, 1985).
Fig. 1.
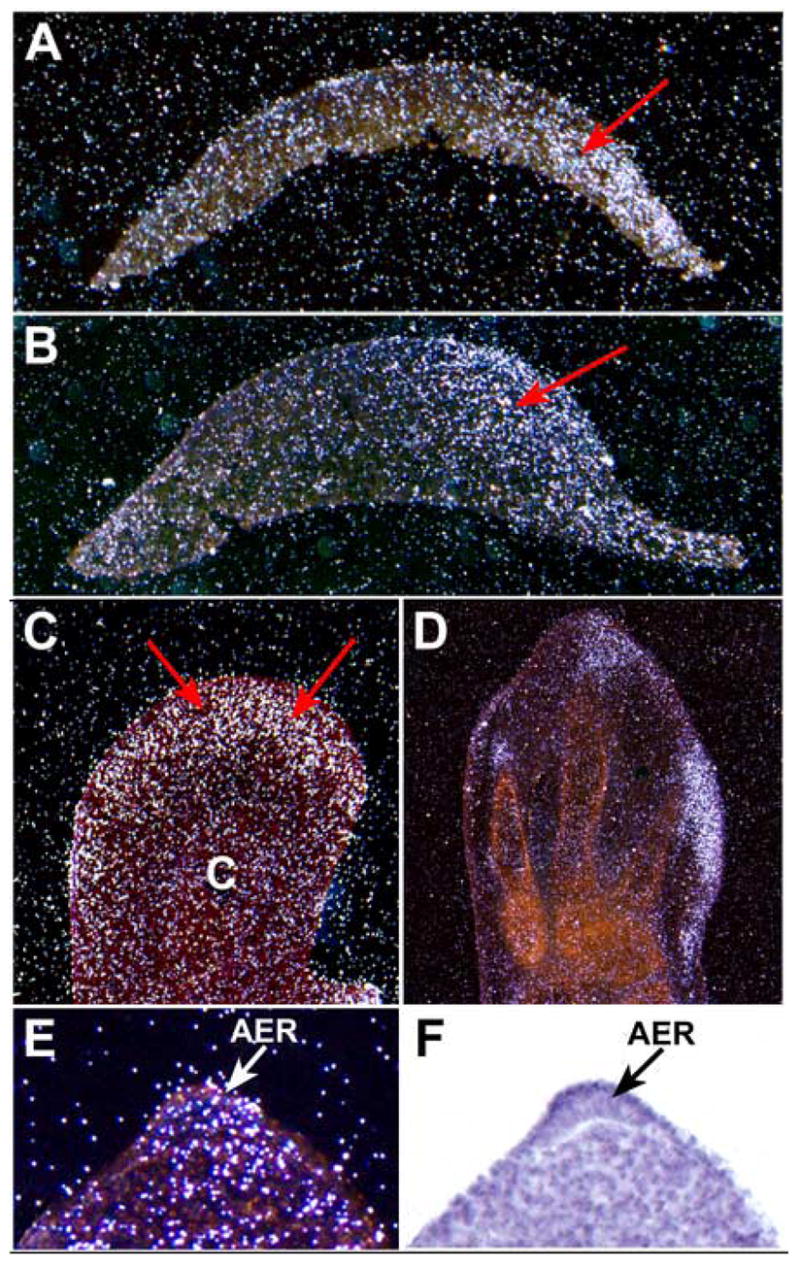
Expression of Has2 during early chick limb bud development analyzed by in situ hybridization. (A–D) Frontal sections through stage 17/18 (A), stage 20 (B), stage 25 (C), and stage 27 (D) limb buds. In each panel the posterior border of the limb bud is on the right and the anterior border on the left. (E, F) Dark field (E) and bright field (F) images of a sagittal section through the AER of a stage 20 limb bud. At stages 17/18 (A), 20 (B), and 25 (C) Has2 is expressed in the distal posterior subridge mesoderm (red arrows). Little or no expression is detectable in the proximal central core (c in panel C) where precartilage condensations are forming. At stage 27 (D) Has2 is expressed in the distal mesoderm at the tips of the digits which is undergoing outgrowth, but is not expressed in the interdigital mesoderm or in the precartilage condensations of the digits. During early limb morphogenesis Has2 is also expressed in the AER (arrows in E, F).
At stage 27, Has2 is expressed at the posterior margin of the autopod and in the distal mesoderm at the tips of the developing digits where outgrowth is occurring in response to the AER (Fig. 1D). In contrast, Has2 is not expressed in the interdigital mesoderm which underlies the portions of the distal ectoderm in which AER activity has ceased (Fig. 1D). Has2 is also not expressed in the precartilage condensations of the developing digits (Fig. 1D).
As shown in Fig. 1E and F, during early limb development, in addition to being expressed in the distal subridge mesoderm, Has2 is also expressed by the AER itself, which is producing relatively high amounts of HA as it is directing the outgrowth, directed migration, and patterning of the underlying mesoderm (Kosher and Savage, 1981). However, little Has2 expression is detectable in nonridge dorsal/ventral limb ectoderm (Fig. 1E,F), which exhibits relatively little HA synthesis (Kosher and Savage, 1981).
Overexpression of Has2 in the mesoderm of the developing chick limb bud in vivo perturbs limb morphogenesis
In a functional study to explore the possible role of HA in early limb development, we overexpressed Has2 via adenoviral and RCAS replication competent retroviral vectors in the mesoderm of the developing chick limb bud in vivo to determine if upregulated and sustained HA production perturbs limb morphogenesis.
As shown in Fig. 2, microinjection of Has2 adenovirus into the mesoderm of the developing limb bud at stage 18, which is shortly after the initial formation of the limb bud, results in the formation of shortened and severely malformed limbs. The phenotype of the Has2 infected limbs is complex and variable, very likely reflecting differences in the extent of infection of different samples as is typical of in vivo misexpression studies (Ferrari et a., 1998; Omi et al., 2005). There are, however, several consistent defects in the Has2 infected limbs. In 42% (11 of 26) of the Has2 infected limbs one or more cartilaginous skeletal elements are completely or partially absent (Fig. 2). Furthermore, 92% (24 of 26) of the Has2 infected limbs are shorter than those of contralateral control limbs (Fig. 2).
Fig. 2.
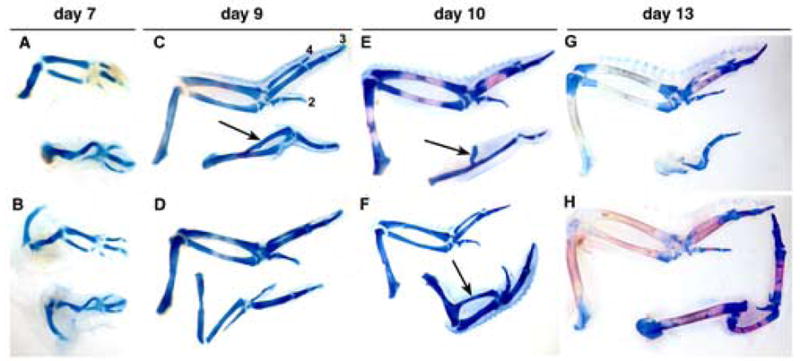
Adenoviral overexpression of Has2 in the mesoderm of the developing chick limb bud in vivo perturbs limb morphogenesis. (A–H) Alcian blue/Alizarin Red-stained skeletal elements of day 7 (A, B), day 9 (C, D), day 10 (E, F), and day 13 (G, H) contralateral control forelimbs (top of each panel) and Has2 infected forelimbs (bottom of each panel) resulting from microinjection of Has2 adenovirus at the onset of limb formation (stage 18). Digits 2, 3, and 4 in the day 9 control limb in panel C are indicated. Has2 infected limbs are shortened (92%) and several (42%) exhibit partial or complete absence of one or more skeletal elements. Skeletal elements that do form in the Has2 infected limbs are often misshapen and exhibit abnormal morphology (65%), and/or are positioned inappropriately (15%). In some cases, a rudimentary skeletal element is fused to and growing out of the shaft of the humerus (arrows in C, E, F), and in some cases (27%) the autopod contains only a digit 3, and digits 4 and 2 are absent.
The skeletal elements of the limbs infected with Has2 adenovirus are not only reduced in length, but also 65% (17 of 26) of the Has2 infected limbs possess skeletal elements that are misshapen and exhibit abnormal morphology (Fig. 2). Indeed, 35% (9 of 26) of the Has2 infected limbs contain skeletal elements that exhibit little resemblance to normal skeletal elements, or are inappropriately arranged or positioned (15%, 4 of 26). In some cases, a rudimentary radius, ulna, or an unidentifiable skeletal element is fused to and growing out of the shaft of the humerus (Fig. 2C, E, F). In 27% (7 of 26) of the Has2 infected limbs the autopod contains only a digit 3, and digits 4 and 2 are absent.
Microinjection of a Has2 RCAS retroviral expression vector into the prospective limb forming region at stage 10 also results in severely shortened and abnormal limbs. In the Has2-RCAS infected limb shown in Fig. 3, all of the segments of the limb are malformed. The stylopod (femur) is severely shortened and misshapen, and the zeugopod consists of a single abnormal skeletal element that articulates with two extremely small digits that bear no resemblance to normal digits (Fig. 3).
Fig. 3.

Alcian blue/Alizarin Red-stained skeletal elements of a day 13 contralateral control hindlimb (top) and Has2 RCAS retrovirus infected hindlimb (bottom) resulting from microinjection of the Has2 retroviral vector into the prospective leg forming region at stage 10. The femur in the Has2 infected limb is shortened and misshapen, and the zeugopod consists of a single abnormal skeletal element that articulates with two extremely small digits that bear no resemblance to normal digits.
Studies on the role of HA in regulation of limb chondrogenesis in vitro
During the initial formation of precartilage condensations in the proximal central core of the developing limb, HA production and Has2 expression are strikingly downregulated and extracellular HA is removed (Kosher et al., 1981; Knudson and Toole, 1985). It has been suggested that this downregulation of HA may be necessary for the close juxtaposition of cells and the critical cell-cell interactions that trigger chondrogenic differentiation during the formation of the precartilage condensations of the skeletal elements (Kosher et al., 1981; Knudson and Toole, 1985). Consistent with this possibility, as described above, we have found that the differentiation of cartilaginous limb skeletal elements is impaired in response to overexpression of Has2 in the mesoderm of the developing chick limb in vivo. In the present study we have investigated this hypothesis further and more directly by determining if sustained HA production in response to retroviral misexpression of Has2 impairs the ability of limb mesenchymal cells to undergo condensation and chondrogenesis in vitro.
In these studies micromass cultures of stage 22–24 limb bud mesenchymal cells were infected for 3 hours at the onset of culture with our Has2 RCAS retroviral expression vector or with a control RCAS retroviral vector lacking the Has2 cDNA inset. Since replication competent RCAS retrovirus needs to be internalized and integrated into the genome of the cells, it should take about 15–24 hours after infection for the Has2 transgene to be expressed. For this reason, in these studies we used micromass cultures established at various relatively low cell densities (2.5–5 × 104 cells/10 μl) at which cartilage differentiation progresses more slowly than in standard high density micromass cultures (2 × 105 cells/10 μl). This was done to ensure that the Has2 transgene would be expressed before or during the formation of precartilage condensations which at these lower densities do not form until day 2 or later after the onset of culture (Rodgers et al., 1989; Seghatoleslami et al., 1996).
As shown in Fig. 4, low density micromass cultures infected with the control RCAS vector form numerous precartilage condensations of closely packed rounded cells detectable by phase contrast microscopy on day 2–3 of culture (Fig. 4A and E). These precartilage aggregates subsequently enlarge and differentiate into cartilage nodules characterized by the deposition of a refractile matrix detectable by phase contrast microscopy (Fig. 4C and G). In contrast, only a few very small condensations form in cultures infected with the Has2 RCAS vector (Fig. 4B and F), and this is subsequently reflected in a striking decease in the number of refractile cartilage nodules that differentiate in the Has2 infected cultures compared to controls (Fig. 4D and H). As shown at higher magnification in Fig. 5, whereas control cultures form numerous precartilage condensations or nodules of closely juxtaposed rounded cells (Fig. 5A), most of the cells of the Has2 infected cultures are large, flattened and spatially separated (Fig. 5B). These results indicate that sustained expression of Has2 inhibits the formation of precartilage condensations and subsequent chondrogenesis.
Fig. 4.
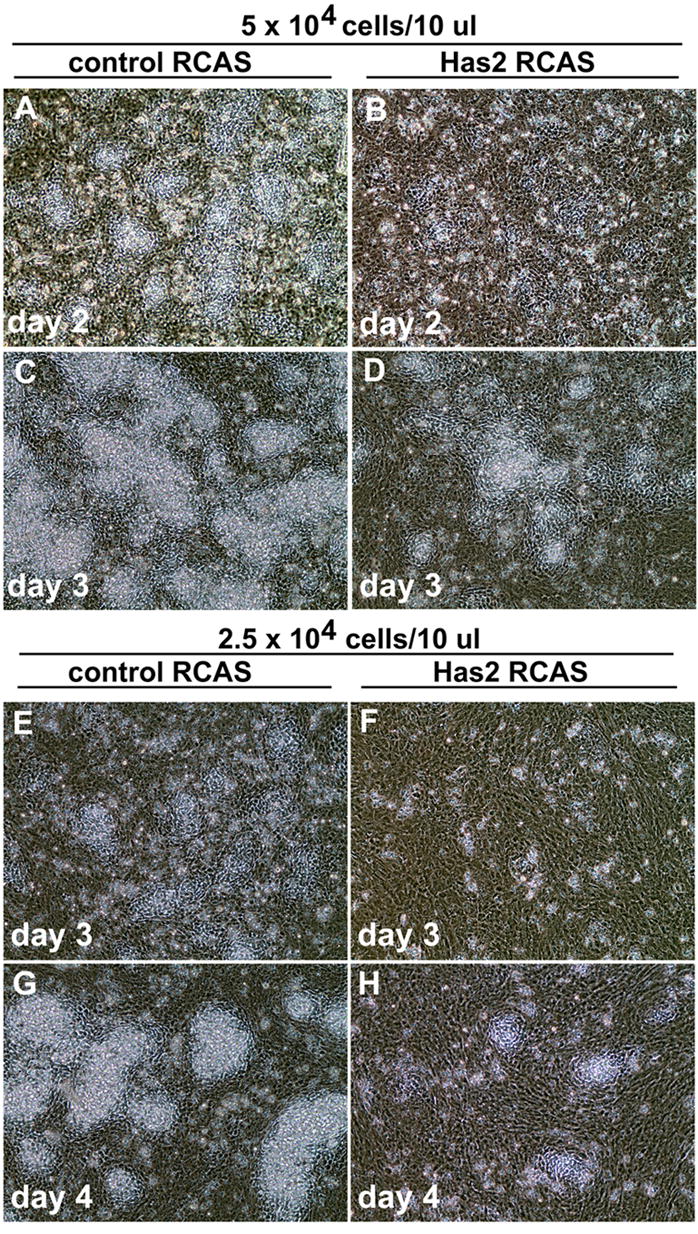
Overexpression of Has2 impairs formation of precartilage condensations and chondrogenesis by limb mesenchymal cells in vitro. (A–H) Phase contrast microscopy images of micromass cultures of stage 22–24 limb bud mesenchymal cells established at 5 × 104 cells/10 μl (A–D) or 2.5 × 104 cells/10 μl (E–H) infected with a control RCAS retrovirus (A,C; E,G) or a Has2 RCAS retrovirus (B,D; F,H). The RCAS control cultures form numerous precartilage condensations of closely packed rounded cells on day 2 (A) or day 3 (E), which subsequently enlarge and differentiate into cartilage nodules which deposit a refractile matrix (C,G). In contrast, only a few very small condensations form in cultures infected with the Has2 RCAS vector (B,F), and there a striking decease in the number of refractile cartilage nodules that differentiate (D,H).
Fig. 5.
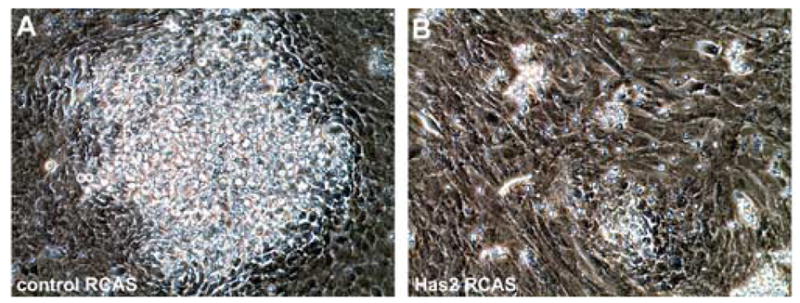
(A,B) Higher magnification phase contrast microscopy images of day 4 limb bud mesenchymal cell micromass cultures established at 2.5 × 104 cells/10 μl and infected for 3 h at the onset of culture with a control RCAS (A) or Has2 RCAS (B) retroviral vector. Control cultures (A) form numerous precartilage condensations or nodules of closely packed rounded cells, whereas most of the cells of Has2 infected cultures (B) remain large, flattened, and spatially separated from one another.
To confirm and quantify the apparent inhibition of chondrogenesis detectable by phase contrast microscopy in the Has2 infected cultures, we examined the accumulation of cartilage matrix in the RCAS control and Has2 RCAS infected cultures by staining with Alcian blue, pH 1.0. As shown in Fig. 6, there is a striking decrease in the extent of Alcian blue staining cartilage matrix in the Has2 infected cultures compared to controls. The area occupied by Alcian blue cartilage matrix as determined by image analysis is reduced by 61–71% in the Has2 infected cultures established at various low cell densities compared to cultures infected with the control RCAS vector (Table 1).
Fig. 6.
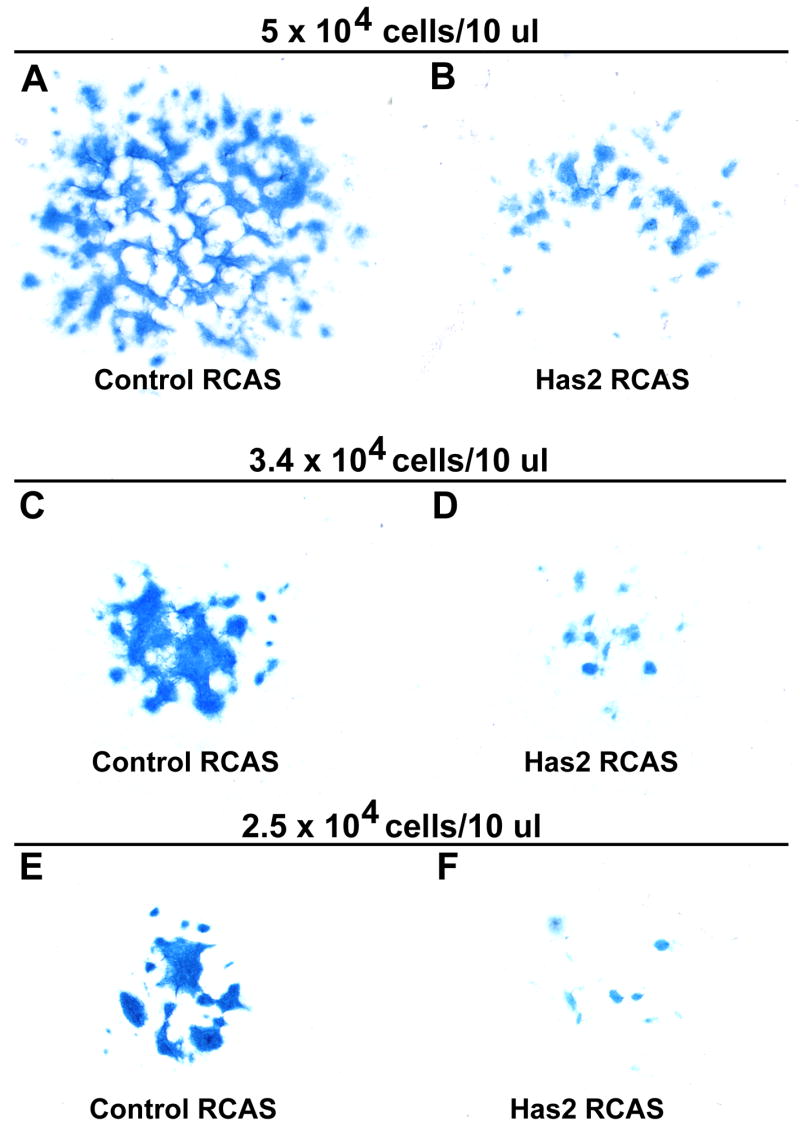
Retroviral overexpression of Has2 inhibits the accumulation of Alcian blue staining cartilage matrix by micromass cultures of limb bud mesenchymal cells established at 5 × 104 cells/10 μl (A,B), 3.4 × 104 cells/10 μl (C,D), or 2.5 × 104 cells/10 μl (E,F). (A–E) Alcian blue-stained day 3 (A,B), day 4 (C,D), and day 6 (E,F) cultures infected with a control RCAS retrovirus (A,C,E) or with a Has2 RCAS retrovirus (B,D,F). There is a striking decrease in the extent of Alcian blue staining cartilage matrix in the Has2 infected cultures (B,D,F) compared to corresponding controls (A,C,E).
Table 1.
Retroviral overexpression of Has2 inhibits the accumulation of Alcian blue staining cartilage matrix by micromass cultures of limb mesenchymal cells established at various cell densities
| Area of culture stained with Alcian blue
|
|||
|---|---|---|---|
| Cell density (per 10 μl) | Treatment | pixels2 (× 103) | Treated/control |
| 5 × 104 | control RCAS | 137 ± 11 | - |
| Has2 RCAS | 54 ± 9 | 0.39 | |
| 3.4 × 104 | control RCAS | 42 ± 7 | - |
| Has2 RCAS | 14 ± 6 | 0.33 | |
| 2.5 × 104 | control RCAS | 28 ± 5 | - |
| Has2 RCAS | 8 ± 2 | 0.29 | |
Values are the means of 8 (5 × 104 control RCAS), 5 (5 × 104 Has2 RCAS), and 4 (3.4 × 104 control RCAS, 3.4 × 104 Has2 RCAS; 2.5 × 104 Has2 RCAS) determinations ± SEM, or 2 determinations ± range (2.5 × 104 control RCAS). Determinations are from day 3 (5 × 104) or day 6 (3.4 × 104 and 2.5 × 104) cultures.
To confirm and examine the extent of ectopic HA production in the Has2 infected cultures, we examined the distribution of HA by histochemical staining with a biotinylated HA binding protein (HABP) which binds strongly and specifically to HA in cells and tissue sections (Knudson and Toole, 1985; Asari et al., 1992; Yu et al., 1996; Shibata et al., 2003; Underhill and Zhang, 2000; Toole et al., 2001). As shown in Fig. 7, relatively little HABP staining is detectable in RCAS control cultures (Fig. 7A), whereas widespread HABP staining is present throughout the Has2 infected cultures (Fig. 7B). No HABP staining is detectable in cultures pretreated with Streptomyces hyaluronidase which specifically degrades HA. These results confirm that there is widespread ectopic HA production in the Has2 RCAS infected cultures in which precartilage condensation and cartilage differentiation is inhibited.
Fig. 7.
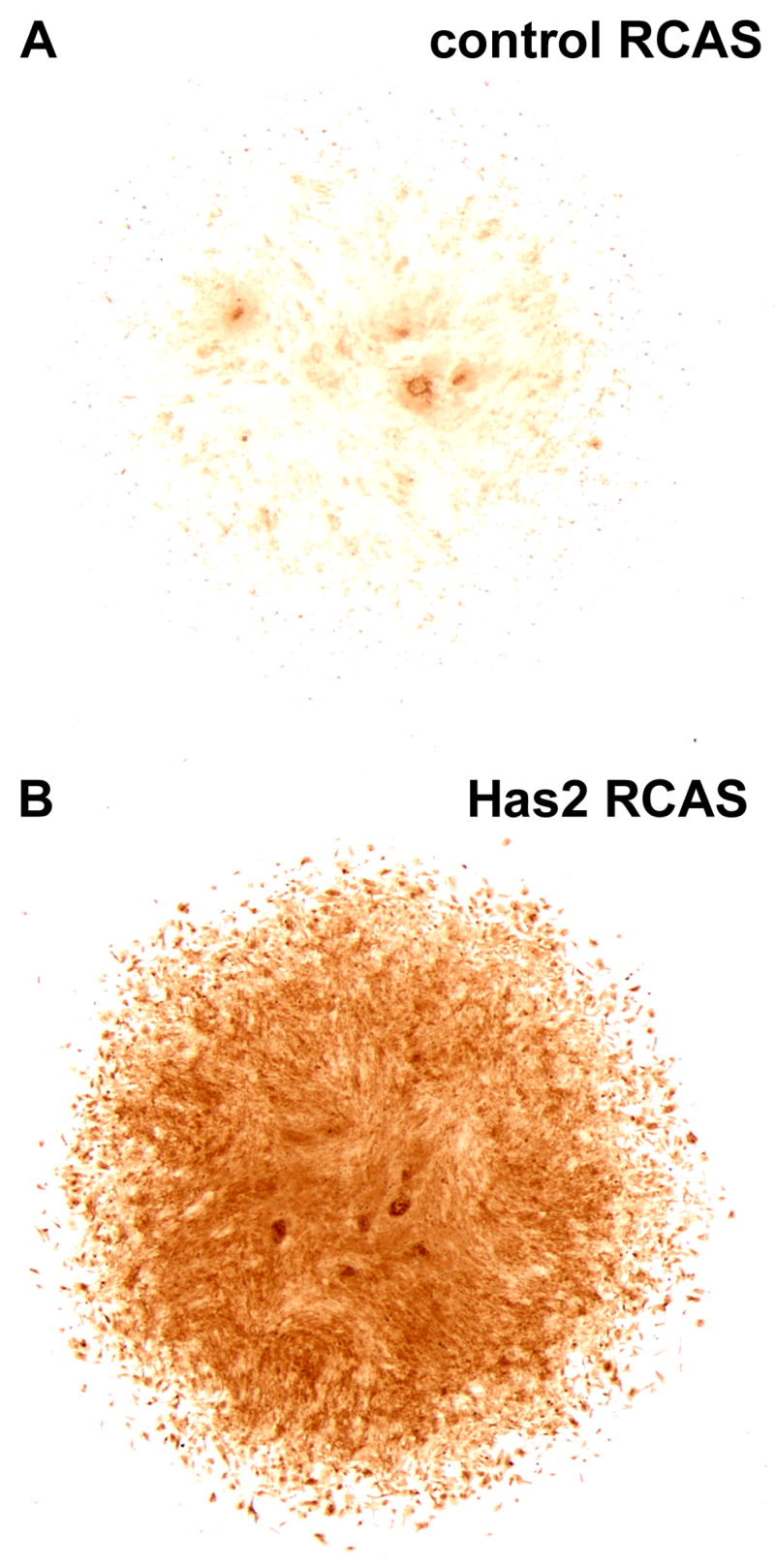
(A, B) Distribution of hyaluronan (HA) in RCAS control (A) and Has2 RCAS (B) infected limb bud mesenchymal cell micromass cultures established at 2.5 × 104 cells/10 μl as assayed by histochemical staining with a biotinylated HA binding protein (HABP). Relatively little HABP staining is detectable in day 5 RCAS control cultures (A), whereas widespread HABP staining is present throughout the Has2 infected cultures (B).
Discussion
HA in Limb Morphogenesis
Consistent with important roles for HA in limb morphogenesis, we have found that overexpression of Has2 in the mesoderm of the chick limb bud in vivo results in the formation of severely malformed limbs. Limbs overexpressing Has2 exhibit complete or partial absence of one or more cartilaginous skeletal elements. This suggests that sustained localized production of HA impairs chondrogenic differentiation, and is consistent with a role for HA in the regulation of the onset of chondrogenesis (see below for further discussion).
Furthermore, overexpression of Has2 results in limbs and skeletal elements that are considerably shorter than normal (92%). This suggests the possibility that HA may be involved in growth of the limb. HA promotes cell proliferation in several model systems (Evanko et al., 1999; Itano et al., 2002; Ward et al., 2003; Rilla et al., 2002), and is present in high amounts in the distal subridge mesoderm of the limb bud which is undergoing directed outgrowth and proliferation in response to the AER. The possible role of HA in limb growth will require further study.
It is also noteworthy that directed proximodistal outgrowth of the limb not only depends on cell proliferation in response to AER signals, but also is facilitated by the directed distal migration of subridge mesenchymal cells towards the AER in response to chemotactic influences (Li et al., 1996; Li and Muneoka, 1999). HA is an important regulator of cell migration in several developing systems (Camenisch et al., 2000; Bakkers et al., 2004; Ori et al., 2005), and can act as a chemotactic signal to promote directional cell migration (Tzircotis et al., 2005).
A relatively high percentage of limbs overexpressing Has2 exhibit patterning defects. In particular, 65% of the Has2 infected limbs have skeletal elements that are misshapen and exhibit abnormal morphology, and 35% have skeletal elements that bear little or no resemblance to normal skeletal elements. Inappropriate positioning of skeletal elements also occasionally occurs. These defects suggest the possibility that HA may be involved in the patterning of the developing limb. Although this possibility will require further study, it is noteworthy that HA is an important co-stimulator of the EGFR/ErbB signaling network, and acts in concert with the ErbB family of tyrosine kinase receptors and ligands to regulate a variety of cellular processes (Camenisch et al., 2002; Tsatas et al., 2002; Bourguigon et al., 1997; Sherman et al., 2000; Ghatak et al., 2005; Misra et al., 2006). This is of particular interest since the EGFR/ErbB signaling network has been implicated in the outgrowth and patterning of the developing limb bud (Omi et al., 2005).
Possible role of HA in AER activity
In the present study we have found that, in addition to being expressed by the distal subridge mesoderm of the developing limb bud, Has2 is also expressed by the AER itself, as the AER is promoting the proliferation and directed migration of the underlying subridge mesenchymal cells. However, little Has2 expression is detectable in nonridge dorsal/ventral limb ectoderm. This observation is consistent with our previous biochemical studies showing that the rate of HA production by the AER is 3–4 fold greater than that of nonridge limb ectoderm (Kosher and Savage, 1981). We suggest that the relatively large amount of HA that is synthesized and secreted by the AER may play an important role in facilitating the interactions between the AER and underlying mesenchymal cells that result in the directed PD elongation of the limb.
The basal lamina subjacent to the AER is diffuse and considerably less well organized than elsewhere in the limb (Tomasek et al., 1980; Newman et al., 1981), and it is likely that the HA produced by the AER contributes to the unique disorganization of the basal lamina, which in turn may be important for facilitating interactions between the AER and underlying mesenchymal cells. The unique disorganization and composition of the AER basal lamina may result in the diminished adherence of the basal surface of AER cells to the diffuse underlying substratum, thus promoting the unique wedge-shaped configuration of the AER which, in turn, creates a sublaminar space in which HA produced by the AER accumulates (Newman et al., 1981). Such a localized HA rich sublaminar space may facilitate directional outgrowth of the mesenchyme by providing a region of low mechanical resistance (Newman et al., 1981). In particular, a localized HA rich sublaminar space may facilitate the directed migration of the mesenchymal cells towards the AER thus encouraging the PD elongation of the limb, and may also facilitate direct contact between the migrating cells and the AER. As mentioned above, HA can act as a chemoattractant, and a chemotactic HA gradient can promote directional cell migration (Tzircotis et al., 2005). Thus, HA synthesized and secreted by the AER may contribute to an HA gradient that promotes the directional migration of the subridge mesenchymal cells towards the AER.
Role of HA in regulation of the onset of limb chondrogenesis
One of the functions of the AER is to maintain the mesenchymal cells directly subjacent to it in a labile undifferentiated condition (Kosher et al., 1979). It has been suggested that the HA rich extracellular matrix produced by the subridge mesenchymal cells may mediate this antidifferentiative effect by keeping the cells largely separated from on another, thus preventing the intimate cell-cell interactions that are necessary to trigger chondrogenic differentiation (Kosher et al., 1981). During the initial formation of precartilage condensations that occurs when the cells in the central core of the limb bud become located outside of the range of AER signaling, Has2 expression and HA production are strikingly downregulated (Kosher et al., 1981; the present study) and concomitantly the HA rich extracellular matrix and pericellular HA rich surface coats are removed (Knudson and Toole, 1985; Kulyk and Kosher, 1987; Nicoll et al., 2002). Thus, it has been suggested that the downregulation of HA production and the removal of extracellular HA may be necessary for the close juxtaposition of cells and the critical cell-cell interactions that trigger chondrogenic differentiation during the formation of precartilage condensations (Kosher et al., 1981). Consistent with this possibility, in the present study we have found that the differentiation of cartilaginous limb skeletal elements is impaired in response to misexpression of Has2 in the mesoderm of the developing chick limb in vivo. Moreover, we demonstrate that sustained production of HA in response to misexpression of Has2 in micromass cultures of limb mesenchymal cells inhibits the formation of precartilage condensations and subsequent chondrogenesis. These results indicate that downregulation of HA is indeed necessary for the formation of the precartilage condensations that trigger cartilage differentiation.
Acknowledgments
This work was supported by NIH grant HD022610.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asira A, Miyauchi S, Miyazaki K, Hamai A, Horie K, Takahashi T, Sekiguchi T, Machida A, Kohno K, Uchiyama Y. Intra- and extracellular localization of hyaluronic acid and proteoglycan constituents (chondroitin sulfate, keratin sulfate, and protein core) in articular cartilage of rabbit tibia. J Histochem Cytochem. 1992;40:1693–1703. doi: 10.1177/40.11.1431058. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Kramer C, Pothof J, Quaedvlieg NEM, Spaink HP, Hammerschmidt M. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development. 2004;131:525–537. doi: 10.1242/dev.00954. [DOI] [PubMed] [Google Scholar]
- Becker TC, Noel RJ, Coats WS, Gomez-Foix AM, Alam T, Gerard RD, Newgard CB. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- Bourguignon LYW, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signaling interactions between CD44 and the transforming growth factor β receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- Bourguignon LYW, Zhu H, Chu A, Iida N, Zhang L, Hung MC. Interaction between the adhesion receptor, CD44, and the oncogene product p185HER2, promotes human ovarian tumor cell activation. J Biol Chem. 1997;272:27913–27918. doi: 10.1074/jbc.272.44.27913. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2–ErbB3 receptors. Nature Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Kelwer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho CND, Sumoy L, Rodgers BJ, Davidson DR, Hill RE, Upholt WB, Kosher RA. Expression of the chicken homeobox-containing gene GHox-8 during embryonic chick limb development. Mech Dev. 1991;34:143–154. doi: 10.1016/0925-4773(91)90051-7. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Kosher RA. Dlx5 is a positive regulator of chondrocyte differentiation during endochondral ossification. Dev Biol. 2002;252:257–270. doi: 10.1006/dbio.2002.0862. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Lichtler A, Pan ZZ, Dealy CN, Upholt WB, Kosher RA. Ectopic expression of Msx-2 in posterior limb mesoderm impairs limb morphogenesis while inducing Bmp-4 expression, inhibiting proliferation and promoting apoptosis. Dev Biol. 1998;197:12–24. doi: 10.1006/dbio.1998.8880. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Li Y, Seghatoleslami MR, Dealy CN, Kosher RA. Heparan sulfate proteoglycans including syndecan-3 modulate BMP activity during limb cartilage differentiation. Matrix Biol. 2006;25:27–39. doi: 10.1016/j.matbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Gakunga P, Frost G, Shuster S, Cunha G, Formby B, Stern R. Hyaluronan is a prerequisite for ductal branching morphogenesis. Development. 1997;124:3987–3997. doi: 10.1242/dev.124.20.3987. [DOI] [PubMed] [Google Scholar]
- Gay SW, Kosher RA. Uniform cartilage differentiation in micromass cultures prepared from a relatively homogeneous population of chondrogenic progenitor cells of the chick limb bud: effect of prostaglandins. J Exp Zool. 1984;232:317–326. doi: 10.1002/jez.1402320219. [DOI] [PubMed] [Google Scholar]
- Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- Gordon LB, Harten IA, Calabro A, Sugumaran G, Csoka AB, Brown WT, Hascall V, Toole BP. Hyaluronan is not elevated in urine or serum in Hutchinson-Gilford Progeria Syndrome. Hum Genet. 2003;113:178–187. doi: 10.1007/s00439-003-0958-9. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Nat Acad Sci USA. 2002;99:3609–3614. doi: 10.1073/pnas.052026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosher RA. The chondroblast and the chondrocyte. In: Hall BK, editor. Cartilage. Vol. 1. Academic Press; New York: 1983. pp. 59–85. [Google Scholar]
- Kosher RA, Savage MP. Glycosaminoglycan synthesis by the apical ectodermal ridge of the chick limb bud. Nature. 1981;291:231–232. doi: 10.1038/291231a0. [DOI] [PubMed] [Google Scholar]
- Kosher RA, Savage MP, Chan SC. In vitro studies on the morphogenesis and differentiation of the mesoderm subjacent to the apical ectodermal ridge of the embryonic chick limb-bud. J Embryol Exp Morph. 1979;50:75–97. [PubMed] [Google Scholar]
- Kosher RA, Savage MP, Walker KH. A gradation of hyaluronate accumulation along the proximodistal axis of the embryonic chick limb bud. J Embryol Exp Morphol. 1981;63:85–98. [PubMed] [Google Scholar]
- Knudson CB, Toole BP. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev Biol. 1985;112:308–318. doi: 10.1016/0012-1606(85)90401-4. [DOI] [PubMed] [Google Scholar]
- Kulyk WM, Kosher RA. Temporal and spatial analysis of hyaluronidase activity during development of the embryonic chick limb bud. Dev Biol. 1987;120:535–541. doi: 10.1016/0012-1606(87)90256-9. [DOI] [PubMed] [Google Scholar]
- Li S, Anderson R, Reginelli AD, Muneoka K. FGF-2 influences cell movements and gene expression during limb development. J Exp Zool. 1996;274:234–247. doi: 10.1002/(SICI)1097-010X(19960301)274:4<234::AID-JEZ4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Li S, Muneoka K. Cell migration and chick limb development: chemotactic action of FGF-4 and the AER. Dev Biol. 1999;211:335–347. doi: 10.1006/dbio.1999.9317. [DOI] [PubMed] [Google Scholar]
- Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- Newman SA, Frisch HL, Perle MA, Tomasek JJ. Limb development: aspects of differentiation, pattern formation and morphogenesis. In: Connelly TJ, editor. Morphogenesis and Pattern Formation. Raven Press; New York: 1981. pp. 163–178. [Google Scholar]
- Nicoll SB, Barrak O, Csoka AB, Bhatnagar RS, Stern R. Hyaluronidases and CD44 undergo differential modulation during chondrogenesis. Biochem Biophys Res Comm. 2002;292:819–825. doi: 10.1006/bbrc.2002.6697. [DOI] [PubMed] [Google Scholar]
- Omi M, Fisher M, Maihle NJ, Dealy CN. Studies on epidermal growth factor receptor signaling in vertebrate limb patterning. Dev Dyn. 2005;233:288–300. doi: 10.1002/dvdy.20353. [DOI] [PubMed] [Google Scholar]
- Ori M, Nardini M, Casini P, Perris R, Nardi I. XHas2 activity is required during somitogenesis and precursor cell migration in Xenopus development. Development. 2005;133:631–640. doi: 10.1242/dev.02225. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Andhare RA, Rousche KT, Knudson W, Wang W, Grossfield JB, Thomas RO, Hollingsworth RE, Knudson CB. CD44 modulates Smad1 activation in the BMP-7 signaling pathway. J Biol Chem. 2004;166:1081–1091. doi: 10.1083/jcb.200402138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilla K, Lammi MJ, Sironen R, Torronen K, Luukkonen M, Hascall VC, Midura RJ, Hyttinen M, Pelkonin J, Tammi M, Tammi R. Changed lamellipodial extension, adhesion plaques and migration in epidermal keratinocytes containing constitutively expressed sense and antisense hyaluronan synthase 2 (Has2) genes. J Cell Sci. 2002;115:3633–3643. doi: 10.1242/jcs.00042. [DOI] [PubMed] [Google Scholar]
- Rodgers BJ, Kulyk WM, Kosher RA. Stimulation of limb cartilage differentiation by cyclic AMP is dependent on cell density. Cell Differentiation Development. 1989;28:179–188. doi: 10.1016/0922-3371(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Saunders JW., Jr The proximo-distal sequence of origin of the parts of the chick wing and the role of ectoderm. J Exp Zool. 1948;108:363–404. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- Sherman LS, Rizvi TA, Karyala S, Ratner N. CD44 enhances neuregulin signaling by Schwann cells. J Cell Biol. 2000;150:1071–1083. doi: 10.1083/jcb.150.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Imai H, Abe T, Yamashita Y. In situ hybridization and immunohistochemistry of versican, aggrecan and link protein, and histochemistry of hyaluronan in the developing mouse limb bud cartilage. J Anat. 2003;203:425–432. doi: 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghatoleslami MR, Kosher RA. Inhibition of in vitro limb cartilage differentiation by syndecan-3 antibodies. Dev Dyn. 1996;207:114–119. doi: 10.1002/(SICI)1097-0177(199609)207:1<114::AID-AJA11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Singley CT, Solursh M. The spatial distribution of hyaluronic acid and mesenchymal cell condensation in the embryonic chick wing. Dev Biol. 1981;84:102–120. doi: 10.1016/0012-1606(81)90375-4. [DOI] [PubMed] [Google Scholar]
- Solursh M. Cell-cell interaction in chondrogenesis. In: Hall BK, editor. Cartilage. Vol. 2. Academic Press; New York: 1983. pp. 121–141. [Google Scholar]
- Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273:1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- Tien JYL, Spicer AP. Three vertebrate hyaluronan synthases are expressed during mouse development in distinct spatial and temporal patterns. Dev Dyn. 2005;233:130–141. doi: 10.1002/dvdy.20328. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, Newman SA. Regional differences in the ultrastructure of the ectodermal basal lamina during amniote limb development. J Cell Biol. 1980;87:120a. [Google Scholar]
- Toole BP. Hyaluronate turnover during chondrogenesis in the developing chick limb and axial skeleton. Dev Biol. 1972;29:321–329. doi: 10.1016/0012-1606(72)90071-1. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. J Int Med. 1997;242:35–40. doi: 10.1046/j.1365-2796.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. Seminars Cell Dev Biol. 2000;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–336. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP, Yu Q, Underhill CB. Hyaluronan and hyaluronan-binding proteins. Probes for specific detection. Methods Mol Biol. 2001;171:479–485. doi: 10.1385/1-59259-209-0:479. [DOI] [PubMed] [Google Scholar]
- Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci. 2002;9:282–288. doi: 10.1054/jocn.2001.1063. [DOI] [PubMed] [Google Scholar]
- Turley RA, Nobel PW, Bourguignon LYW. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Tzircotis G, Thorne RF, Isacke CM. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J Cell Sci. 2005;118:5119–5128. doi: 10.1242/jcs.02629. [DOI] [PubMed] [Google Scholar]
- Underhill CB, Zhang L. Analysis of hyaluronan using biotinylated hyaluronan-binding proteins. Methods Mol Biol. 2000;137:441–447. doi: 10.1385/1-59259-066-7:441. [DOI] [PubMed] [Google Scholar]
- Ward JA, Huang L, Guo H, Ghatak S, Toole BP. Perturbation of hyaluronan interactions inhibits malignant properties of glioma cells. Am J Pathol. 2003;162:1403–1409. doi: 10.1016/S0002-9440(10)64273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–14000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- Yu Q, Grammatikakis N, Toole BP. Expression of multiple CD44 isoforms in the apical ectodermal ridge of the embryonic mouse limbs. Dev Dyn. 1996;207:204–214. doi: 10.1002/(SICI)1097-0177(199610)207:2<204::AID-AJA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278:45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]


