Abstract
Hypertension may impact pelvic arterial blood flow resulting in reduction of nitric oxide synthase (NOS) levels. Although doxazosin, an α1-adrenoceptor antagonist, has been shown to improve erectile dysfunction as well as benign prostatic hyperplasia (BPH) and hypertension, it is not clear whether these improvements using doxazosin are primarily due to direct actions on the prostate, urinary bladder and penis, possibly via inhibition of vascular α1-adrenoceptors, or other sites of actions. Therefore, we investigated effects of doxazosin to the spontaneously hypertensive rat (SHR) on blood flow and NOS levels in the genitourinary tract. Four groups of rats were assessed: group 1, SHRs treated with doxazosin (30 mg/kg/day) for 4 weeks; group 2, SHRs treated with nifedipine (30 mg/kg/day) for 4 weeks; group 3, untreated SHRs; and group 4, untreated Wistar-Kyoto (WKY) rats. Blood flow to the ventral prostate, dorsolateral prostate, urinary bladder and penis was determined using a fluorescent microsphere infusion technique. Expression levels of nNOS and eNOS mRNAs were quantified by real-time RT-PCR using SYBR Green I. Blood flow to the ventral prostate, dorsolateral prostate, urinary bladder and penis was significantly lower in untreated SHRs than WKY rats. Treatment with doxazosin increased blood flow to each tissue studied in SHRs. RT-PCR data indicated that untreated SHRs had lower mRNA expression levels of nNOS in the bladder and penis and eNOS in the penis than WKY rats and that administration of doxazosin to the SHR caused an increase in expression levels of these genes, i.e., up-regulation of nNOS in the bladder and penis and eNOS in the penis. However, nifedipine had no significant effects on blood flow and NOS levels in the SHR genitourinary tract. Our data demonstrate that doxazosin treatment causes differential alterations in blood flow and NOS levels in the SHR genitourinary tract. These findings may provide insight into the beneficial effects of α1-adrenoceptor antagonists, on prostate, bladder and penile function, when used to treat symptoms of BPH and elevated blood pressure.
Keywords: Doxazosin, Blood flow, Nitric oxide synthase, Rat
Introduction
Erectile dysfunction, benign prostatic hyperplasia (BPH) and hypertension occur with increasing prevalence with advancing age, and often occur concomitantly (Kaplan et al., 2006), suggesting a common cause rather than independent age-related changes.
Hypertension may impact pelvic arterial blood flow resulting in loss of smooth muscle in the bladder with resultant loss of bladder compliance (Tarcan et al., 1998). Simultaneously, prostate ischemia results in prostate fibrosis with resultant increase in urethral resistance (Tarcan et al., 1998). These changes observed in the bladder and prostate result in lower urinary tract symptoms. Similarly, penile ischemia results in loss of smooth muscle in the penis with resultant erectile dysfunction (Tarcan et al., 1998). Furthermore, pelvic ischemia may reduce nitric oxide synthase (NOS) expression in the prostate, urinary bladder and penis (McVary, 2005). This proposed reduction in NOS isoforms results in decreased smooth muscle relaxation.
The clinical trial data demonstrate that doxazosin, an α1-adrenoceptor antagonist, not only relieves the symptoms of BPH, i.e., lower urinary tract symptoms, and reduces blood pressure in patients with hypertension, but also improves erectile dysfunction of patients with and without those concomitant conditions (Kaplan et al., 2006). However, it is not clear whether these improvements using doxazosin are primarily due to direct actions on the prostate, urinary bladder and penis, possibly via inhibition of vascular α1-adrenoceptors, or other sites of actions. Therefore, in this communication we used spontaneously hypertensive rats (SHRs) to test the hypothesis that oral administration of doxazosin to a hypertensive animal model may cause an increase in blood flow and an up-regulation in NOS levels in the prostate, urinary bladder and penis.
Materials and methods
Animals
Male SHR and the genetically normotensive control strain, the Wistar-Kyoto (WKY) rat, aged 10 weeks were used. The rats were distributed initially in four groups: group 1, 10 SHRs received doxazosin (30 mg/kg/day) orally for 4 weeks; group 2, 10 SHRs received nifedipine (30 mg/kg/day) orally for 4 weeks; group 3, 10 SHRs received the vehicle (5% DMSO); and group 4, 10 WKY rats received the vehicle (5% DMSO). The dosage and duration of treatments were based on consultation with Pfizer who has extensive experience in the treatment of rats with doxazosin.
The mean arterial pressure was measured twice in basal resting conditions by the tail-cuff method using an IITC Co. computerized Model 31 system. The rats were kept in a temperature-controlled chamber at 28.5°C for 15 min before these determinations.
Fluorescent microsphere infusion
Blood flow to the ventral prostate, dorsolateral prostate, urinary bladder and penis was determined using a fluorescent microsphere infusion technique as previously described (Das et al., 2002). Microspheres lodge in perfused tissue and can be used to measure instantaneous blood flow to a tissue via a comparison to the number of spheres in the blood. A saline suspension of FluoSpheresR fluorescent microspheres (Invitrogen, Tokyo, Japan) was infused through the catheter into the carotid artery, while a reference blood sample specimen was simultaneously obtained from each rat through the femoral artery. After the completion of the microsphere infusion procedure, rats were sacrificed, and blood samples and tissue specimens were used for quantification of fluorescent microspheres.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Using cDNA prepared by RT of RNA extracted from frozen tissues, expression levels of nNOS and eNOS mRNAs were quantified by real-time RT-PCR using SYBR Green I as previously described (Yono et al., 2002). Sequences of oligonucleotides used as primers for nNOS, eNOS, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are summarized in table 1. Immediately after the amplification, melt curve protocols were performed to ensure that primer-dimers and other non-specific products had been minimized or eliminated.
Table 1.
Sequences of oligonucleotides used as primers
| mRNA | Sequence (5′ → 3′) | Lengtha | Accession No.b | |
|---|---|---|---|---|
| nNOS | sense | TGGCAACAGCGACAATTTGA | 71 | NM_052799 |
| antisense | CACCCGAAGACCAGAACCAT | |||
| eNOS | sense | CCGGCGCTACGAAGAATG | 79 | NM_021838 |
| antisense | CAGTGCCACGGATGGAAATT | |||
| β-actin | sense | AGATGACCCAGATCATGTTTGAGA | 86 | NM_031144 |
| Antisense | ACCAGAGGCATACAGGGACAA | |||
| GAPDH | sense | GCCAGCCTCGTCTCATAGACA | 75 | NM_017008 |
| antisense | TGGTAACCAGGCGTCCGATA |
Amplicon length in base pairs
Genbank accession number of cDNA and corresponding gene, available at http://www.ncbi.nlm.nih.gov/.
Data analyses
Quantification of fluorescent microspheres in each blood sample and tissue specimen was performed as previously described (Das et al., 2002). The microspheres are too large to pass through the capillary bed, and they lodge into the tissues into which they circulate. Therefore, the microspheres in the tissue specimens were compared to the microspheres found in the reference blood specimen obtained via the femoral catheter from the same rat to calculate the blood flow to each tissue as milliliters per minute per gram tissue.
RT-PCR data were analyzed with the iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA) as previously described (Yono et al., 2002). In brief, an initial copy number of the target gene was calculated from its standard curve and normalized against an initial copy number of the housekeeping gene, which also was calculated from its standard curve.
One-way analysis of variance was performed among the groups, and, where differences were found, Scheffé’s F-test was performed for analyses of significance. P < 0.05 was considered to be statistically significant.
Results
Conscious untreated SHRs had higher mean arterial pressure than normotensive WKY rats (Table 2). Although treatment with antihypertensive drugs, doxazosin and nifedipine, significantly reduced mean arterial pressure in SHRs, it remained elevated compared to that in WKY rats (Table 2). Although our tissue weight data show a relatively high S.E.M. of the results from 10 rats (Table 2), these data are consistent with previous studies (Foster et al., 2004; Yono et al., 2004, 2006). The tissue weights of the genitourinary tract in untreated SHRs were similar to those in WKY rats, and the drugs tested had no significant effects on the tissue weights in SHRs (Table 2).
Table 2.
Blood pressures and genitourinary tissue weights of experimental animals
| WKY |
SHR |
|||
|---|---|---|---|---|
| Untreated | Untreated | Doxazosin | Nifedipine | |
| Mean arterial pressure, mmHg | 118 ± 3 | 175 ± 6a | 138 ± 5a,b | 132 ± 4a,b |
| Tissue weight, mg | ||||
| Ventral prostate | 402 ± 18 | 421 ± 26 | 392 ± 32 | 401 ± 17 |
| Dorsolateral prostate | 287 ± 22 | 312 ± 39 | 322 ± 28 | 308 ± 24 |
| Urinary bladder | 101 ± 10 | 121 ± 18 | 98 ± 21 | 113 ± 13 |
| Penis | 280 ± 33 | 276 ± 31 | 300 ± 33 | 282 ± 26 |
SHRs were treated with doxazosin or nifedipine for 4 weeks. Each value represents the mean ± S.E.M. of the results from 10 rats.
Significantly different from comparable values for WKY rats
Significantly different from comparable values for untreated SHRs.
Blood flow to the ventral prostate, dorsolateral prostate, urinary bladder and penis was significantly lower in untreated SHRs than WKY rats (Fig. 1). Nifedipine had no significant effects on blood flow, whereas treatment with doxazosin caused a significant 1.5-fold increase in blood flow to each tissue studied in SHRs (Fig. 1). Thus, blood flow to each tissue was not significantly different between doxazosin-treated SHRs and WKY rats (Fig. 1).
Fig. 1. Blood flow in the rat ventral prostate, dorsolateral prostate, urinary bladder and penis.
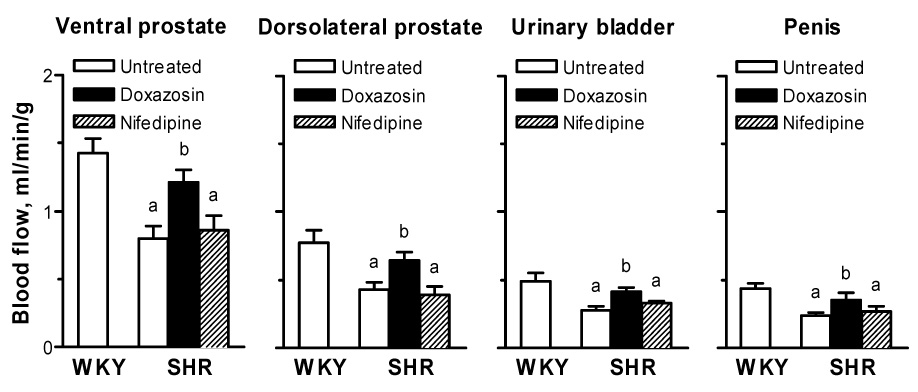
SHRs were treated with doxazosin or nifedipine for 4 weeks. Each bar represents the mean ± S.E.M. of the results from 5 rats.
aSignificantly different from comparable values for WKY rats; bSignificantly different from comparable values for untreated SHRs.
The expression levels of β-actin and GAPDH mRNAs had similar distribution profiles in the rat genitourinary tract. Relative expression of GAPDH mRNA levels normalized against β-actin showed a low variability between the tissues studied (data not shown), suggesting that they are suitable housekeeping genes for normalization.
The relative expression levels of nNOS and eNOS mRNAs normalized against β-actin in rat tissues are shown in Fig. 2. RT-PCR data indicated that both nNOS and eNOS were expressed in all tissues studied and that expression levels of these genes were the highest in the penis among all tissues. Untreated SHRs had lower mRNA expression levels of nNOS in the bladder and penis, but not in the prostate, and eNOS only in the penis than WKY rats. Nifedipine had no significant effects on NOS levels, whereas administration of doxazosin to the SHR caused a significant 3-4-fold increase in expression of these genes, i.e., up-regulation of nNOS in the bladder and penis and eNOS in the penis. However, expression levels of these genes were significantly lower in doxazosin-treated SHRs than WKY rats. Similar expression profiles of nNOS and eNOS mRNAs in the rat ventral prostate, dorsolateral prostate, urinary bladder and penis were obtained with data normalized against GAPDH (data not shown).
Fig. 2. Expression of nNOS and eNOS mRNAs in the rat ventral prostate, dorsolateral prostate, urinary bladder and penis.
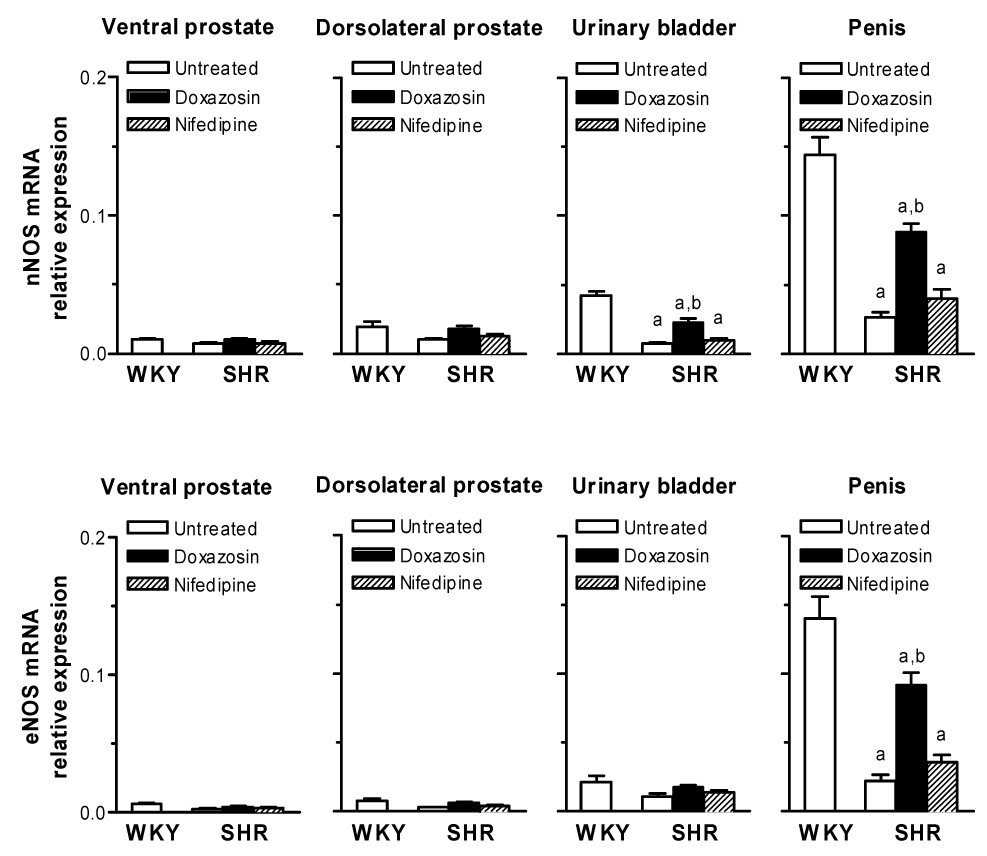
SHRs were treated with doxazosin or nifedipine for 4 weeks. Relative expression levels of mRNA are normalized against β-actin. Each bar represents the mean ± S.E.M. of the results from 5 rats.
aSignificantly different from comparable values for WKY rats; bSignificantly different from comparable values for untreated SHRs.
Discussion
The present study demonstrates that oral administration of doxazosin, but not nifedipine, to a hypertensive animal model causes a significant increase in blood flow to the prostate, bladder and penis and a significant increase in mRNA expression levels of nNOS in the bladder and penis and eNOS in the penis. These findings suggest that α1-adrenoceptor antagonists may contribute to the improvement of lower urinary tract symptoms and erectile dysfunction by inhibiting the vascular α1-adrenoceptors and facilitating the nitric oxide (NO)-induced smooth muscle relaxation in the genitourinary tract.
The SHRs have been widely studied as a genetic model of hypertension. Several studies have demonstrated that SHRs develop prostatic hyperplasia, bladder hyperactivity and erectile dysfunction (Persson et al., 1998; Steers et al., 1999; Golomb et al., 2000; Hale et al., 2002). In the present study, untreated SHRs had lower blood flow to the prostate, bladder and penis than normotensive WKY rats. Chronic ischemia/hypoxia resulting from decreased blood flow induces fibrosis and reduces NOS expression in the SHR genitourinary tract (Tarcan et al., 1998; McVary, 2005). These changes that influence smooth muscle relaxation in the prostate, bladder and penis may play an important role in inducing lower urinary tract symptoms and erectile dysfunction in a hypertensive animal model.
There are considerable variations in the expression levels of nNOS and eNOS in various tissues (Seyam et al., 1999). Our RT-PCR data show that both nNOS and eNOS were expressed in all tissues studied and that expression levels of these genes were the highest in the penis among all tissues. NO plays a fundamental role in the maintenance of the smooth muscle tone of the genitourinary tract (Burnett, 1995). NO synthesized by nNOS appears to be an important factor in the relaxation of the bladder and prostatic smooth muscle (Burnett, 1995), involved in micturition. In the penis, NO synthesized by nNOS and eNOS is the mediator of penile erection (Burnett, 1995; Lugg et al., 1995). Furthermore, the age-related iNOS induction in the penis would prevent penile fibrosis (Gonzalez-Cadavid and Rajfer, 2005). However, further investigations are needed to conclusively evaluate the effects of iNOS induction. α1-Adrenoceptors on peripheral vessels have been found to mediate vasoconstrictor response postsynaptically. In the present study, unlike nifedipine, a calcium channel antagonist, doxazosin increased pelvic blood flow. In some arterial vessels, except in aorta, noradrenaline-induced contractile responses in a calcium-free medium increased with age and were further increased in SHRs compared to WKY rats (Miquel et al., 2005), suggesting that internal calcium stores mobilized by α1-adrenergic stimulus increases with age, especially in SHRs. Pelvic arteries in SHRs may be more sensitive to pharmacological blockade of α1-adrenoceptors via oral antagonists than to calcium channel blockade. Thus, it is conceivable that differential alterations in NOS levels in the SHR genitourinary tract, which could be due to increased pelvic blood flow resulting from inhibiting the vascular α1-adrenoceptors, may contribute to the improvement of lower urinary tract symptoms and erectile dysfunction. It is to be noted, however, that α1-adrenoceptor antagonists with excessive hypotensive effects may hinder erectile function by reducing perfusion of the penis.
Rabbit penile arteries have a predominant, functional α1A-adrenoceptor population with little evidence of other α1-adrenoceptor subtypes (Morton et al., 2007). This finding may inform the design of drugs to assist penile function. However, the highly selective α1A-adrenoceptor antagonist, Ro70-0004, did not improve erectile function when compared to placebo (Choppin et al., 2001). The arterial supply to the prostate, bladder and penis is normally derived from branches of the internal iliac arteries. Rudner and associates (1999) have shown that all three α1-adrenoceptor subtype mRNAs are expressed in rat iliac artery. Furthermore, these authors demonstrated age-related increases in mammary artery α1-adrenoceptor density and a switch from α1A predominance in younger adults to α1B>α1A in older patients. Thus, it is conceivable that subtype nonselective antagonists such as doxazosin may be more effective to increase in pelvic arterial blood flow.
Among α1-adrenoceptor antagonists used for the treatment of BPH, doxazosin has also been employed for the treatment of hypertension. Basic science evidence has defined important roles of the α1-adrenoceptors in the progression of cardiovascular disease through regulation of metabolism, apoptosis and hypertrophy in addition to regulation of hemodynamic responses (Shannon and Chaudhry, 2006). In a large clinical study, however, the use of doxazosin was associated with a high risk of stroke and combined cardiovascular disease events, particularly congestive heart failure (ALLHAT Collaborative Research Group, 2000). Considering the extensive use of α1-adrenoceptor antagonists for the treatment of BPH, further studies on the role of α1-adrenoceptors in heart failure will be needed to determine the therapeutic effect of pharmacological blockade of α1-adrenoceptors.
In conclusion, our data suggest that doxazosin treatment causes differential alterations in blood flow and NOS levels in the SHR genitourinary tract. These findings may provide insight into the beneficial effects of α1-adrenoceptor antagonists, on prostate, bladder and penile function, when used to treat symptoms of BPH and elevated blood pressure.
Acknowledgements
This study was supported in part by NIH grants DK 38311 and DK 42530. All the animal studies were approved by Institutional Animal Care and Use Committee, Kumamoto University and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Journal of the American Medical Association. 2000;283(15):1967–1975. [PubMed] [Google Scholar]
- Burnett AL. Nitric oxide control of lower genitourinary tract functions: a review. Urology. 1995;45(6):1071–1083. doi: 10.1016/s0090-4295(99)80136-8. [DOI] [PubMed] [Google Scholar]
- Choppin A, Blue DR, Hegde SS, Gennevois D, McKinnon SA, Mokatrin A, Bivalacqua TJ, Hellstrom WJ. Evaluation of oral Ro70-0004/003, an α1A-adrenoceptor antagonist, in the treatment of male erectile dysfunction. International Journal of Impotence Research. 2001;13(3):157–161. doi: 10.1038/sj.ijir.3900653. [DOI] [PubMed] [Google Scholar]
- Das AK, Leggett RE, Whitbeck C, Eagen G, Levin RM. Effect of doxazosin on rat urinary bladder function after partial outlet obstruction. Neurourology and Urodynamics. 2002;21(2):160–166. doi: 10.1002/nau.10045. [DOI] [PubMed] [Google Scholar]
- Foster HE, Jr, Yono M, Shin D, Takahashi W, Pouresmail M, Afiatpour P, Latifpour J. Effects of chronic administration of doxazosin on α1-adrenoceptors in the rat prostate. Journal of Urology. 2004;172(6 Pt 1):2465–2470. doi: 10.1097/01.ju.0000138475.89790.88. [DOI] [PubMed] [Google Scholar]
- Golomb E, Rosenzweig N, Eilam R, Abramovici A. Spontaneous hyperplasia of the ventral lobe of the prostate in aging genetically hypertensive rats. Journal of Andrology. 2000;21(1):58–64. [PubMed] [Google Scholar]
- Gonzalez-Cadavid NF, Rajfer J. The pleiotropic effects of inducible nitric oxide synthase (iNOS) on the physiology and pathology of penile erection. Current Pharmaceutical Design. 2005;11(31):4041–4046. doi: 10.2174/138161205774913372. [DOI] [PubMed] [Google Scholar]
- Hale TM, Okabe H, Bushfield TL, Heaton JP, Adams MA. Recovery of erectile function after brief aggressive antihypertensive therapy. Journal of Urology. 2002;168(1):348–354. [PubMed] [Google Scholar]
- Kaplan SA, De Rose AF, Kirby RS, O’leary MP, McVary KT. Beneficial effects of extended-release doxazosin and doxazosin standard on sexual health. BJU International. 2006;97(3):559–566. doi: 10.1111/j.1464-410X.2005.05959.x. [DOI] [PubMed] [Google Scholar]
- Lugg JA, Gonzalez-Cadavid NF, Rajfer J. The role of nitric oxide in erectile function. Journal of Andrology. 1995;16(1):2–4. [PubMed] [Google Scholar]
- McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. European Urology. 2005;47(6):838–845. doi: 10.1016/j.eururo.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Miquel R, Gisbert R, Serna E, Perez-Vizcaino F, Anselmi E, Noguera MA, Ivorra MD, D'Ocon MP. Acute and chronic captopril, but not prazosin or nifedipine, normalize alterations in adrenergic intracellular Ca2+ handling observed in the mesenteric arterial tree of spontaneously hypertensive rats. Journal of Pharmacology and Experimental Therapeutics. 2005;313(1):359–367. doi: 10.1124/jpet.104.078725. [DOI] [PubMed] [Google Scholar]
- Morton JS, Daly CJ, Jackson VM, McGrath JC. α1A-Adrenoceptors mediate contractions to phenylephrine in rabbit penile arteries. British Journal of Pharmacology. 2007;150(1):112–120. doi: 10.1038/sj.bjp.0706956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K, Pandita RK, Spitsbergen JM, Steers WD, Tuttle JB, Andersson KE. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. American Journal of Physiology. 1998;275(4 Pt 2):R1366–R1373. doi: 10.1152/ajpregu.1998.275.4.R1366. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular α1-adrenergic receptors by vessel bed and age. Circulation. 1999;100(23):2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Seyam RM, Huynh HT, Brock GB. Neuronal and endothelial nitric oxide synthase isoforms: quantification of protein and mRNA in the normal rat penis. International Journal of Impotence Research. 1999;11(6):301–308. doi: 10.1038/sj.ijir.3900451. [DOI] [PubMed] [Google Scholar]
- Shannon R, Chaudhry M. Effect of α1-adrenergic receptors in cardiac pathophysiology. American Heart Journal. 2006;152(5):842–850. doi: 10.1016/j.ahj.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Steers WD, Clemow DB, Persson K, Sherer TB, Andersson KE, Tuttle JB. The spontaneously hypertensive rat: insight into the pathogenesis of irritative symptoms in benign prostatic hyperplasia and young anxious males. Experimental Physiology. 1999;84(1):137–147. doi: 10.1111/j.1469-445x.1999.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Tarcan T, Azadzoi KM, Siroky MB, Goldstein I, Krane RJ. Age-related erectile and voiding dysfunction: the role of arterial insufficiency. British Journal of Urology. 1998;82(Suppl 1):26–33. doi: 10.1046/j.1464-410x.1998.0820s1026.x. [DOI] [PubMed] [Google Scholar]
- Yono M, Takahashi W, Pouresmail M, Johnson DR, Foster HE, Jr, Weiss RM, Latifpour J. Quantification of endothelins, their receptors and endothelin-converting enzyme mRNAs in rat genitourinary tract using real-time RT-PCR. Journal of Pharmacological and Toxicological Methods. 2002;48(2):87–95. doi: 10.1016/s1056-8719(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Yono M, Foster HE, Jr, Shin D, Takahashi W, Pouresmail M, Latifpour J. Doxazosin-induced up-regulation of α1A-adrenoceptor mRNA in the rat lower urinary tract. Canadian Journal of Physiology and Pharmacology. 2004;82(10):872–878. doi: 10.1139/y04-098. [DOI] [PubMed] [Google Scholar]
- Yono M, Foster HE, Jr, Weiss RM, Latifpour J. Age related changes in the functional, biochemical and molecular properties of α1-adrenoceptors in the rat genitourinary tract. Journal of Urology. 2006;176(3):1214–1219. doi: 10.1016/j.juro.2006.04.038. [DOI] [PubMed] [Google Scholar]


