Abstract
Frequent or severe abnormal behavior may be associated with the release of endorphins that positively reinforce the behavior with an opiate euphoria or analgesia. One line of research exploring this association involves the superhormone, proopiomelanocortin (POMC). The products of POMC appear to be dysregulated in some human subjects who exhibit self-injurious behavior (SIB). Macaque monkeys have POMC very similar to humans, and some laboratory macaques display SIB or frequent stereotypies. We investigated associations between plasma levels of three immunoreactive POMC fragments with possible opioid action and abnormal behavior ratings in macaques. In 58 adult male and female macaques (24 Macaca fascicularis and 34 M. nemestrina), plasma levels of intact beta-endorphin (βE) and the N-terminal fragment (BEN) were significantly higher in animals with higher levels of abnormal behavior. The C-terminal fragment (BEC) was significantly higher in males but unrelated to ratings of abnormal behavior. Levels of ACTH, cortisol, and (βE-ACTH)/βE dysregulation index were unrelated to abnormal behavior. None of the POMC products differed significantly by subjects' species, age, or weight. The finding that intact beta-endorphin is positively related to abnormal behavior in two species of macaque is consistent with some previous research on human subjects and nonprimates. The positive relation of the N-terminal fragment of βE to abnormal behavior is a new finding.
Keywords: Self-injurious behavior (SIB), stereotypy, abnormal behavior, opioids, beta-endorphin, proopiomelanocortin (POMC), adrenocorticotropic hormone (ACTH), cortisol, Macaca fascicularis, Macaca nemestrina
Introduction
Severe behavioral disorders, including self-injurious behavior (SIB) and frequent stereotypies, can compromise the well-being of humans and of captive animals [22,46,49]. Once established, abnormal behaviors can become intractable to treatment. The persistence of behaviors that interfere with normal life, in some cases causing pain and suffering, has inspired various hypotheses. The self-stimulating properties of stereotypies may develop to increase arousal in unstimulating environments; or stereotyped behavior may occur during states of increased stress, serving as a coping mechanism by dissipating tension, frustration or anxiety; or both [13,47]. The long-term maintenance of abnormal behavior may result if the individual displaying it is somehow rewarded through its exhibition [34].
One hypothesis for the manifestation of frequent or severe abnormal behavior is that it is associated with the release of endorphins that positively reinforce the behavior with an opiate euphoria [44]. A related hypothesis – the pain hypothesis – is based on the idea that behaviorally released or atypically high levels of endogenous opioids increase the pain threshold through analgesia [44,53]. The role of endogenous opioids in behavior has been investigated using opioid antagonists, or blockers, such as naloxone and naltrexone, which eliminate the potentially rewarding properties of euphoria and pain attenuation [3,30,35]. For example, analgesic effects were reversed (i.e., pain thresholds were lowered) by naloxone in most animal studies [3]. Experiments with rats have shown that the exogenous opioid morphine can activate a part of the brain that mediates the hedonic (reward) experience while making the animal less sensitive in another area of the brain that subserves pain [20].
A study of pigs found an association between stereotypies and the release of endorphins: injections of naloxone temporarily reduced stereotypies in tethered sows but exerted no effect on movements associated with normal exploratory behavior of unrestrained sows [11]. Inhibition of stereotypies by opioid antagonists was also reported for poultry, voles, and horses [21]. Opiate blockers reduce SIB in humans, being most effective in patients with elevated levels of endorphin [42,44]. Naltrexone reduced the frequency and severity of SIB in male rhesus macaques (Macaca mulatta) with a history of severe self-wounding [36]. Rhesus macaques with a history of SIB directed self-biting preferentially toward body areas associated with acupuncture analgesia [26]. This association also has been reported in human subjects [50]. Collectively, these data are consistent with the hypotheses that SIB and stereotypies might be positively reinforced and maintained by the pleasurable sensations or analgesic properties associated with the release of endogenous opioids.
One line of research exploring the role of opioids and abnormal behavior involves the cleaving of the superhormone, proopiomelanocortin (POMC). POMC and its numerous hormonal products and receptors influence a wide range of physiological functions [16]. POMC produces several behaviorally active products including the opioid beta-endorphin (βE) and adrenocorticotropic hormone (ACTH), which stimulates the synthesis of cortisol. These products of POMC appear to be dysregulated in some humans who exhibit SIB, including self-inflicted tissue damage and potential injury. This “uncoupling,” expressed as a “dysregulation index,” results in different ratios of ACTH and opioids in the bloodstream, especially under conditions of stress [45]. Humans with “contagious” SIB, where bouts of SIB follow one another in rapid succession, have especially high levels of intact βE relative to ACTH [45]. Rhesus macaques with high rates of SIB have blunted cortisol responses to mild stress [31,52]. Autistic individuals, with and without SIB, have higher ACTH and lower cortisol basal levels compared to controls [12]. HPA dysregulation has been observed among adults with a history of post-traumatic stress disorder [17,57].
Previous research has identified three POMC products with possible opioid activity, the highly specific “intact” βE component (βE1-31), a smaller sequence (βE1-23), and another fragment that is highly cross-reactive with beta-lipotropin (β-LP1-91) [44]. These latter fragments are sometimes referred to as N-terminal and C-terminal, respectively, because of their proximity to the H2N terminus and COOH terminus peptides surrounding the supermolecule, POMC [48]. In research on humans, two of the immunoreactive fragments (C-terminally directed and intact βE) have been reported to be dysregulated at rest among autistic individuals, with and without SIB [44]. The third fragment (N-terminal-directed) has not been reported to differ among SIB patients. Some autistic subjects with SIB were reported to have normal ACTH levels but elevated levels of the C-terminally directed βE-immunoreactivity relative to the N-terminally directed immunoreactivity [23,24]. In another study, autistic subjects were found to have markedly elevated C-terminal βE concentrations compared to normal individuals, but did not differ significantly in N-terminal βE concentrations; six of the ten subjects had a history of mild SIB [4]. The possible opioid activity of the third fragment was suggested by an analysis of beta-endorphin fragment activity on human monocyte chemotaxis indicating that the N-terminal, but not the C-terminal, has opiate effects [39].
Some laboratory primates display SIB or frequent stereotypies. As a consequence, they may experience diminished psychological well-being, and the research projects in which they participate may be compromised [1,14,31,32]. Recent data [53] contribute to our understanding of the potential role of endogenous opioids in the development and maintenance of severe abnormal behavior in nonhuman primates but do not address the contribution of different βE fragments and are restricted to rhesus macaques (Macaca mulatta). High sequence homologies in POMC across a variety of mammalian species and vertebrates in general [19,48] support comparisons between human and nonhuman primates. Of particular relevance to the current research is the strong similarity in βE: The sequence of the last 31 amino acids coded by the POMC gene, corresponding to βE1-31, is identical for M. mulatta and M. nemestrina, and the first 30 of these are identical to Homo sapiens; the only difference is the last amino acid, in the C-terminal region [29,33] [NCBI Entrez Protein Database Accession Numbers P01201, XP_001082999, NP_000930].
In this study, we investigated associations between POMC fragments and abnormal behavior ratings in two laboratory monkey species, longtailed macaques (M. fascicularis) and pigtailed macaques (M. nemestrina). Significant associations with abnormal behavior were used to assess opioid activity of the products investigated. We investigated in these nonhuman primates plasma levels of three different immunoreactive POMC fragments with possible opioid action: intact βE (BET-IR, further abbreviated to BET), N-terminally directed βE-immunoreactivity (BEN-IR, or BEN), and C-terminally directed βE/βLP (beta-lipotropin) immunoreactivity (BEC-IR, or BEC). Our goals were to determine if ratings of severity of abnormal behavior correlate with intact βE and other POMC products or with dysregulation between βE and ACTH, and whether levels of the POMC products differ by species or sex.
1. Methods
1.1. Subjects and sample collection
Venous blood samples were drawn from 58 adult macaques (24 Macaca fascicularis, and Mf, 34 Macaca nemestrina, Mn) under ketamine sedation between 8:10 AM and 12:30 PM (most between 9:30 and 11:30 AM). Blood was collected into EDTA tubes containing aprotinin, a serine protease inhibitor. The plasma was stored at −70 degrees C until assayed in the lab of A. Chicz-DeMet.
We requested research support staff to collect samples from similar numbers of adult males and females and at least 20 per species. Subjects were scheduled for routine blood draw for other research projects over a three-month period and were not selected for this study on the basis of behavioral or other criteria. The number of subjects drawn per day varied, as did the interval between sedation and venipuncture. Eleven male and 11 female Mf were subjects in a long-term study of prenatal exposure to mercury (10 controls, 12 dosed) [15]. The mercury subjects did not differ significantly from their undosed control subjects in any analysis in this study. All subjects were housed in individual cages at the National Primate Research Center (NPRC) at the University of Washington, Seattle, an Institutional Animal Care and Use Committee (IACUC)-approved and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility with a fully implemented Environmental Enhancement Plan, as required by law [55].
1.2. POMC Peptide Assays
β-Endorphin Assay (intact βE1-31 immunoreactivity, BET-IR, or BET)
Plasma levels of BET-IR were determined by a commercially available direct solid phase two-site immunoradiometric assay (IRMA) using antibodies against synthetic human βE (Nichols Institute Diagnostics; San Juan Capistrano, CA). Cross-reactivity of pertinent analytes is summarized in Table 1. The BET IRMA method measures intact βE1-31 in a radiolabeled soluble sandwich complex bound to two antibodies with high affinity and specificity for βE coupled to a solid bead matrix. Briefly, samples (200 μl) were incubated with an antibody-coated bead and labeled antiserum (100 μl) for 20±1 hours at room temperature. The labeled antibody complex bound to the solid phase was measured using an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. All plasma βE values were calculated using a four-parameter logistics program [37] obtained from a modified standard curve enriched with two additional low-level standards. The calculated assay sensitivity was approximately 5.7 pg/ml. Intra- and inter-assay coefficients of variation (CVs) were 4.2% and 8.3%, respectively.
Table 1.
Crossreactivity (%) with POMC Fragments for Beta-Endorphin Directed Antisera for POMC-Peptide Assays
| Peptide | C-terminal / βLP assaya |
N-terminal assaya |
Specific βE1-31 assayb |
|---|---|---|---|
| BEC-IR | BEN-IR | BET-IR | |
| βE1-31 | 100 | 100 | 100 |
| β-Lipotropin1-91 | 102 | <1.0 | 16 @ 500 pg/ml |
| βE6-31 | 84 | 2 | N/A |
| βE1-27 | 75 | 43 | 0.007 @ 5mg/ml |
| βE1-17 | N/A | N/A | 0.007 @ 5mg/ml |
| βE1-16 | <0.01 | 69 | 0.005 @ 5mg/ml |
| Leu-enkephalin | <0.01 | <0.04 | <0.001 @ 5mg/ml |
| Met-enkephalin | <0.01 | 9 | <0.001 @ 5mg/ml |
Directional insert; Euro-Diagnostica AB, Malmo, Sweden
Directional insert; Nichols Institute Diagnostics, San Juan Capistrano, CA
N/A not available
β-Endorphin N-terminally directed immunoreactivity (BEN-IR, or BEN) and β-Endorphin C-terminally directed immunoreactivity (BEC-IR, or BEC)
Three ml of plasma were extracted with acetone activated Sep-pak C18 cartridge (Waters, Milford, MA) and eluted with acidified acetone (1.5 ml of 0.2 M HCl/acetone (25:75). The extracts were dried under vacuum and reconstituted in assay buffer. BEN-IR and BEC-IR were measured by competitive radioimmunoassays (RIAs) employing antisera directed at human BEN and BEC (Euro-Diagnostica AB, Malmo, Sweden). These assays specifically detect human BEN (<1% cross-reactivity with βLP) and human BEC (102% cross-reactivity with βLP), with non-significant cross-reactivity with ACTH and related opiate analogs as listed in Table 1. Reconstituted samples were incubated (100 μl/assay tube) with antiserum (200 μl) for 24 hr at 4°C, followed by a second overnight incubation with 125I-βE (200 μl) at 4°C. Labeled and unlabeled βE was collected by immunoprecipitation and the aspirated pellets were quantified using a gamma counter (ICN Biomedical, formerly Micromedic, Isoflex Gamma Counter). BEN-IR and BEC-IR levels were calculated using a standard curve computed by a four-parameter logistics program [37] corrected for dilution. The detection limits of the BEN-IR and BEC-IR RIAs are 10 pg/ml. For BEN-IR and BEC-IR, respectively, the intra-assay CVs were 5.2% and 9.1% and the inter-assay CVs were 17.6% and 21.6%.
Adrenocorticotropin Hormone (ACTH) Assay
Plasma levels of ACTH were measured directly in plasma by an IRMA method using human ACTH antibodies with non-significant cross-reactivity with βE and ACTH fragments, and with reported detection limits of 1.0 pg/ml (Nichols Institute Diagnostics; San Juan Capistrano, CA). Briefly, samples (200 μl) combined with ACTH labeled antibody (100 μl) and a coated bead were incubated at room temperature for 20±1 hours. The bound radiolabeled antibody complex was quantified using an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. Concentrations of ACTH were determined directly from the standard curve computed by a four-parameter logistics program [37]. The intraand inter-assay CVs were 4.4% and 10.8%, respectively.
Plasma Cortisol Assay
Plasma cortisol levels were determined by a competitive antibody coated tube RIA with reported sensitivity of 0.21 μg/dL (GammaCoat™, DiaSorin, Stillwater, MN). Plasma samples (10 μl) were incubated with 125I-labeled cortisol (1.0 ml) in antibody-coated tubes for 45 min in a 37°C water bath. The aspirated antibody-bound labeled tubes were counted on an ICN Biomedical (formerly Micromedic) Isoflex Gamma Counter. Cortisol concentrations were interpolated from a standard curve computed by a four-parameter logistics program [37]. Cross-reactivities of the cortisol assay were 6.3% with 11-deoxycortisol, <1.5% with cortisone, prednisone, corticosterone, and <0.1% with other naturally occurring steroids. The intra- and inter-assay CVs were 6.6% and 10.6%, respectively.
1.3. Abnormal behavior ratings
Abnormal behavior ratings were determined by staff in the Psychological Well-being (PWB) Program at the Washington National Primate Research Center (WaNPRC). Raters were blind to assay results. Behavioral records were summarized in a rating scale (Table 2). The rating scale ranged from 1 = no abnormal behavior to 15 = self-inflicted injury sufficient to break the skin that was the closest in time to the date of blood draw. The subjects were assigned the rating that corresponded to the most serious abnormal behavior(s) recorded for the animal.
Table 2.
Abnormal behavior ratings
| Rating | Abnormal behavior category |
|---|---|
| 1 | No abnormal behavior detected. Never referred to Psychological Well- being Program staff for abnormal behavior. Behavioral observations by trained staff support absence of abnormal behavior. |
| 2 | Never referred; no other information; no SIB (defined as self-injury sufficient to break the skin) in animal's clinical record. |
| 3 | Low levels of abnormal behavior (excluding self-injurious behavior – SIB - or potentially self-injurious behavior); e.g., infrequent locomotor stereotypy, overgrooming, feces smearing, self-clasping. Low levels defined as >0 to <15% of time budget spent engaging in abnormal behavior or observed in ≤25% of behavioral assessment visits. Half of the 58 subjects were rated 3 or below (median split). |
| 4 | Minor levels of abnormal behavior (without any self-injurious or potentially self-injurious behavior). Minor levels defined as 15% to <20% of time budget spent engaging in abnormal behavior or observed in >25% to ≤50% of behavioral assessment visits. |
| 5 | Minor or low levels of abnormal behavior that include potentially self- injurious behavior (e.g., self-biting or self-hitting that does not break the skin); no SIB in animal's clinical record. |
| 6 | Frequent abnormal behavior (primarily frequent locomotor stereotypy such as pacing) but no SIB or potentially self-injurious behavior. Frequent defined as ≥20% of time budget spent engaging in abnormal behavior or observed in >50% of behavioral assessment visits. |
| 7 | Frequent abnormal behavior including any level of potentially self-injurious behavior; no SIB in animal's clinical record. Example: animals that self-bite without injury and also engage in frequent locomotor stereotypy. |
| 8-15 | Animals with history of injury-producing SIB, ratings ordered by temporal proximity to POMC blood collection: 8, the animal with its last SIB episode most distant in time, to 15, the animal with SIB episodes around the time of POMC blood collection. |
As part of its role in overseeing behavioral well-being of laboratory primates, the PWB Program compiles data on abnormal behavior of monkeys. The list of subjects was provided to PWB staff in the absence of assay results. Existing behavioral data were searched, and at least four observations were made on remaining subjects with no relevant behavioral information. For subjects that had been referred to the PWB Program for assessment of abnormal behavior, the occurrence or absence of abnormal behaviors from a defined list was scored since referral during weekly assessment visits. Some of these referred animals also had been scored repeatedly during systematic 10-minute behavioral assessment observations [1]. The clinical animal records of all 58 subjects were searched for evidence of self-wounding. Some monkeys were housed individually but had grooming contact with a partner through widely spaced bars [9] and were observed systematically once a week to assess ongoing compatibility irrespective of having been referred for abnormal behaviors. Although the focus was on social behaviors, any abnormal behaviors were also noted. Most of the remaining subjects were observed systematically at least four times by PWB staff to record the occurrence or absence of abnormal behaviors. Fourteen subjects, deceased before the observations could be completed, were assigned rating 2 (Table 2). Subjects with behavioral information and no reports of abnormal behavior were assigned rating 1 (Table 2).
Eight of the 58 subjects had a history of self-inflicted injury sufficient to break the skin (PWB definition of SIB); some injuries were serious enough to be recorded in the animal's clinical record. SIB is the most concerning abnormal behavior to PWB staff. These 8 subjects were given unique ratings of 8 through 15 based on the temporal proximity of SIB to the date of blood draw. PWB behavioral definitions require breaking of the skin for self-directed behavior to be classified as self-injurious behavior (SIB); behavior such as self-biting or self-hitting that does not break the skin is classified by PWB as potential SIB. In the human literature, SIB definitions generally include behaviors that can potentially cause tissue damage, but may not necessarily break the skin [4, p. 193 includes examples]. In our rating scale, infrequent abnormal behavior including low levels of potential SIB was rated 5, below frequent stereotypy without potential SIB (rating 6) and rating 7: frequent abnormal behavior including any level of noninjurious self-biting or other potentially self-injurious behavior, but no history of actual SIB (Table 2).
We chose this rating method because it combines measures of severity and frequency across all types of abnormal behaviors into a continuous scale. For statistical analyses, each abnormal behavior rating was converted to an Abnormality Rank (AbnRank) score (minimum rank = 7.5, the average rank of 15 animals with tied ratings of “1”; maximum rank = 58, the rank of the subject rated 15).
1.4. Statistical tests
The peptide measures analyzed include plasma levels of: BET (BET-IR pg/ml), BEC (BEC-IR pg/ml), BEN (BEN-IR pg/ml), ACTH (pg/ml), and Cortisol (μg/dl). A dysregulation index, DYSBET = ((BET-ACTH)/BET) × 100, was computed to characterize the degree of uncoupling between BET and ACTH and to eliminate sources of variation inherent in measures of absolute concentration [42]. When BET and ACTH are equal, the index equals zero. A positive index means that BET is elevated above ACTH, and a negative index means that ACTH is elevated above BET.
Statistical analyses were performed using SPSS (Spearman correlations; Univariate Analysis of Variance), and Data Desk 6.2 (General Linear Model ANOVA-MANCOVA, Type 3, Partial; Fisher exact tests) [56]. Two-tailed probabilities of ≤ .05 are reported as statistically significant.
2. Results
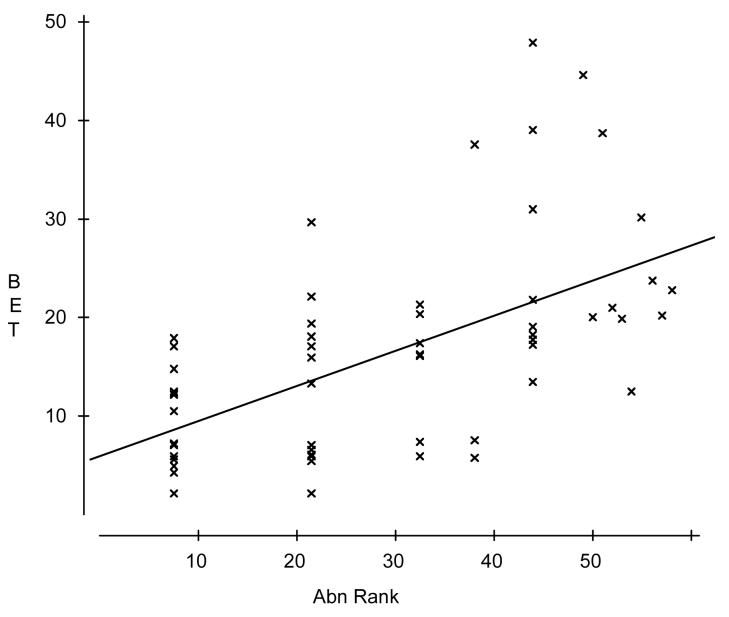
Table 3 summarizes the means and SDs for all dependent variables and the independent variable AbnRank by species-sex category and across all samples. Univariate ANOVAs found no significant differences between mercury-dosed monkeys and their controls on any dependent variable or AbnRank, so their data were combined with the remaining subjects. Across all 58 subjects, AbnRank was strongly positively correlated with plasma levels of intact beta-endorphin (BET), moderately correlated with BEC, BEN, and DYSBET, and weakly correlated with ACTH and (negatively) with cortisol (Table 4). A concordant pattern of significance for both species and both sexes held only for the positive correlation between plasma levels of BET and AbnRank (Figure 1). Correlations between AbnRank and ACTH or Cortisol were significant only when all 58 subjects were included. Other correlations only were significant for one sex and/or species.
Table 3.
Summary statistics for dependent and independent variables
| Variable | Spec-Sex | N | Mean | StdDev | Min | Max |
|---|---|---|---|---|---|---|
| AbnRank | all | 58 | 29.5 | 16.59 | 7.5 | 58 |
| AbnRank | Mf-F | 10 | 28.8 | 15.05 | 7.5 | 56 |
| AbnRank | Mf-M | 14 | 39.93 | 15.62 | 7.5 | 57 |
| AbnRank | Mn-F | 19 | 17.11 | 11.81 | 7.5 | 53 |
| AbnRank | Mn-M | 15 | 35.93 | 14.41 | 7.5 | 58 |
| BET (pg/ml) | all | 58 | 16.40 | 10.59 | 2.07 | 47.86 |
| BET (pg/ml) | Mf-F | 10 | 17.70 | 6.94 | 7.01 | 29.64 |
| BET (pg/ml) | Mf-M | 14 | 25.85 | 11.36 | 12.33 | 47.86 |
| BET (pg/ml) | Mn-F | 19 | 8.51 | 5.29 | 2.07 | 19.78 |
| BET (pg/ml) | Mn-M | 15 | 16.69 | 9.71 | 5.73 | 44.65 |
| BEN (pg/ml) | all | 58 | 51.43 | 15.16 | 32.79 | 114.24 |
| BEN (pg/ml) | Mf-F | 10 | 55.94 | 10.16 | 43.87 | 81.34 |
| BEN (pg/ml) | Mf-M | 14 | 55.73 | 12.81 | 33.27 | 83.12 |
| BEN (pg/ml) | Mn-F | 19 | 42.41 | 5.95 | 32.79 | 53.12 |
| BEN (pg/ml) | Mn-M | 15 | 55.84 | 22.51 | 34.99 | 114.24 |
| BEC (pg/ml) | all | 58 | 66.64 | 45.66 | 22.83 | 233.70 |
| BEC (pg/ml) | Mf-F | 10 | 56.54 | 25.55 | 25.49 | 120.24 |
| BEC (pg/ml) | Mf-M | 14 | 116.30 | 64.15 | 27.16 | 233.70 |
| BEC (pg/ml) | Mn-F | 19 | 39.30 | 10.91 | 22.83 | 64.05 |
| BEC (pg/ml) | Mn-M | 15 | 61.67 | 22.68 | 39.41 | 117.41 |
| DYSBET | all | 58 | −386.12 | 311.37 | −1625.76 | 26.10 |
| DYSBET | Mf-F | 10 | −341.17 | 298.20 | −1138.03 | −128.21 |
| DYSBET | Mf-M | 14 | −292.09 | 171.96 | −722.11 | −35.59 |
| DYSBET | Mn-F | 19 | −577.47 | 403.66 | −1625.76 | −91.80 |
| DYSBET | Mn-M | 15 | −261.45 | 154.87 | −545.79 | 26.10 |
| ACTH (pg/ml) | all | 58 | 62.90 | 44.57 | 18.85 | 317.52 |
| ACTH (pg/ml) | Mf-F | 10 | 66.03 | 21.03 | 32.59 | 95.44 |
| ACTH (pg/ml) | Mf-M | 14 | 100.38 | 73.00 | 30.50 | 317.52 |
| ACTH (pg/ml) | Mn-F | 19 | 42.58 | 10.44 | 27.54 | 75.49 |
| ACTH (pg/ml) | Mn-M | 15 | 51.56 | 24.60 | 18.85 | 123.90 |
| Cortisol (μg/dl) | all | 58 | 37.70 | 10.09 | 14.49 | 61.17 |
| Cortisol (μg/dl) | Mf-F | 10 | 34.96 | 10.77 | 14.49 | 55.41 |
| Cortisol (μg/dl) | Mf-M | 14 | 34.12 | 11.01 | 18.16 | 61.17 |
| Cortisol (μg/dl) | Mn-F | 19 | 41.07 | 8.38 | 23.64 | 56.98 |
| Cortisol (μg/dl) | Mn-M | 15 | 38.59 | 10.11 | 22.31 | 59.63 |
| AGE (yr) | all | 58 | 13.55 | 4.48 | 5 | 20 |
| AGE (yr) | Mf-F | 10 | 18.40 | 0.97 | 17 | 19 |
| AGE (yr) | Mf-M | 14 | 16.00 | 3.55 | 7 | 19 |
| AGE (yr) | Mn-F | 19 | 12.74 | 3.83 | 7 | 20 |
| AGE (yr) | Mn-M | 15 | 9.07 | 2.34 | 5 | 13 |
| Weight (kg) | all | 58 | 8.85 | 4.43 | 3 | 19.4 |
| Weight (kg) | Mf-F | 10 | 3.69 | 0.38 | 3 | 4.1 |
| Weight (kg) | Mf-M | 14 | 6.31 | 0.92 | 5.2 | 8.8 |
| Weight (kg) | Mn-F | 19 | 8.76 | 2.05 | 5.9 | 14.5 |
| Weight (kg) | Mn-M | 15 | 14.79 | 3.20 | 6 | 19.4 |
Table 4.
Spearman Rank Correlations between AbnRank, POMC fragments, and cortisol
| AbnRank vs. | All | pa | Mf | p | Mn | p | Males | p | Females | p |
|---|---|---|---|---|---|---|---|---|---|---|
| BET****b | 0.632 | *** | 0.611 | ** | 0.49 | ** | 0.522 | ** | 0.507 | ** |
| BEC | 0.49 | *** | 0.268 | NS | 0.54 | ** | 0.334 | NS | 0.187 | NS |
| BEN | 0.496 | *** | 0.244 | NS | 0.398 | * | 0.507 | ** | 0.201 | NS |
| DYSBETc | 0.45 | *** | 0.091 | NS | 0.553 | *** | 0.166 | NS | 0.471 | * |
| ACTH | 0.314 | * | 0.341 | NS | 0.087 | NS | 0.265 | NS | 0.092 | NS |
| CORTISOL | −0.285 | * | −0.229 | NS | −0.251 | NS | −0.136 | NS | −0.229 | NS |
| n of subjects | 58 | 24 | 34 | 29 | 29 |
* p <.05, ** p < .01, *** p < .001,two-tailed (bold)
**** Concordant pattern across species and sexes (bold)
DYSBET=((BET−ACTH)/BET) × 100
Figure 1.
Plasma levels of intact beta-endorphin (BET, pg/ml) increased significantly with abnormal behavior rank (Abn Rank). Spearman correlation = +.63 (p < .001, n=58).
To further investigate factors contributing to the significant correlations, we ran GLM-MANCOVAs with AbnRank as a continuous independent variable, where higher ranks reflected more severe behavior disorders, and with sex (M, F) and species (Mf, Mn) as discrete factors. Because the species and sexes varied in weight, and the mercury project subjects were older than most others, weight and age at time of blood draw were also included as continuous covariates. The first MANCOVA included the beta-endorphin variables (Table 5a: BET, BEC, BEN). The second MANCOVA included the dysregulation, ACTH, and Cortisol variables (Table 5b: DYSBET, ACTH, Cortisol). AbnRank significantly predicted plasma levels of BET and BEN (positive relation), but BEC was unrelated to abnormal behavior rank (Table 5a). Sex was significantly related to BEC (levels higher in males) and approached significance for BET (Table 5a). No independent variables (Abnormal Rank, Sex, Species, Age, Weight) significantly predicted levels of the dependent variables DYSBET, ACTH, or Cortisol (Table 5b). Species, weight, and age were not significantly associated with any of the dependent variables (DV).
Table 5.
GLM-MANCOVAsa overview of p-values with percentage of unique variance accounted for by independent variable (factor) given in parentheses (%UniqV).
| a. Dependent variables Intact Beta-endorphin (BET), N-Terminal (BEN), and C-Terminal (BEC) | ||||
|---|---|---|---|---|
| Source (factor) | Wilks | BET (%UniqV) | BEN (%UniqV) | BEC (%UniqV) |
| AbnRank | 0.007 | 0.01 (6.7) | 0.03 (6.1) | 0.55 (0) |
| Sex | 0.053 | 0.06 (2.8) | 0.82 (0) | 0.01 (8.5) |
| Species | 0.49 | 0.42 (0) | 0.46 (0.7) | 0.56 (0) |
| Weight | 0.66 | 0.84 (0) | 0.55 (0) | 0.62 (0) |
| Age | 0.64 | 0.19 (0.8) | 0.73 (0) | 0.18 (1.1) |
| Regression adjusted R-squared | 43.3% | 14.0% | 35.3% | |
| b. Dependent variables DYSBET, ACTH or Cortisoly | ||||
| Source (factor) | Wilks | DYSBET (%UniqV) | ACTH (%UniqV) | Cortisol (%UniqV) |
| AbnRank | 0.11 | 0.10 (3.0) | 0.64 (0) | 0.15 (2.0) |
| Sex | 0.07 | 0.61 (0) | 0.15 (1.7) | 0.51 (0) |
| Species | 0.11 | 0.40 (0) | 0.46 (0) | 0.15 (2.1) |
| Weight | 0.38 | 0.29 (0.3) | 0.69 (0) | 0.70 (0) |
| Age | 0.06 | 0.6 (0) | 0.37 (0) | 0.30 (0.2) |
| Regression adjusted R-squared | 15.2% | 18.7% | 5.0% | |
df = 1, 52 for each factor. Bold indicates p ≤ .05. Wilks's Lambda probability indicates significance of factor across all DVs.
Abnormal behavior rank was used as the DV in GLM-ANCOVA. AbnRank differed significantly by sex (males more abnormal) (F1,53 = 6.40, p < .02), but not by species, weight or age (all p > .36). Nonparametric statistics also found males to be significantly more likely to have an abnormality rank above the median (abnormal behavior ratings 4-15, Table 2) (Fisher's exact test, p < .0001, 2-tailed). Comparing species, Mf and Mn did not differ significantly in abnormality rank above the median (Fisher's exact test, p = .17, 2-tailed).
In summary, while simultaneously controlling for the effects of abnormal behavior rank, sex, species, weight, and age, GLM-MANCOVA found that plasma levels of intact βE (BET) and the N-terminally directed immunoreactive fragment (BEN) were both significantly higher in animals with higher levels of abnormal behavior. Plasma levels of the C-terminally directed immunoreactive fragment (BEC) was significantly higher in males and unrelated to abnormal behavior rank in these macaques. Males had higher levels of abnormal behavior rankings than females, but the GLM controls for sex differences in abnormal behavior. The dysregulation index DYSBET, ACTH, and cortisol did not vary significantly by sex or AbnRank. Plasma levels of none of the POMC products varied significantly by species, age, or weight of subjects.
3. Discussion
3.1 The relation between abnormal behavior and different beta-endorphin fragments
This study characterizes in nonhuman primates three different immunoreactive POMC fragments with possible opioid action. Multivariate analyses identified significant factors while controlling for the effects of others. The finding that intact βE (BET) is positively related to abnormal behavior rank scores in two species of macaque is consistent with previous research on nonprimates associating stereotypy and endorphins [11,21]. However, the significant positive relation of BEN, but not BEC, to abnormal behavior is a new finding. In the monkeys studied here, the N-terminal fragment (BEN) is significantly related to the overall abnormal behavior rank score, but not to sex. The C-terminal fragment (BEC) is significantly related to a monkey's sex (higher levels in males) and unrelated to their abnormal behavior. This suggests that, in these monkeys, intact βE and the N-terminal, but not the C-terminal, have similar opioid activity.
The macaque results differ from those reported for some autistic humans with SIB, whose plasma levels of the C-terminal fragment are elevated relative to the N-terminal fragment [23,24]. The Leboyer group found that naltrexone responses in autistic humans suggested opioid activity in the C-terminal fragment but not the N-terminal. In subjects whose autistic symptoms were markedly reduced under low-dose naltrexone treatment, their normally elevated plasma levels of C-terminal βE were also reduced to normal levels under naltrexone [4]. The N-terminal assay found no significant differences in plasma levels between autistic subjects and controls, nor any changes under naltrexone versus baseline conditions.
These contrasting results may be due in part to differences in assays. Many assays have been reported in the literature for measuring POMC products, and they are not necessarily comparable. The profile of cross-reactivity for the three antisera used in the present study (Table 1) indicates that all three antibodies we used to detect these fragments are highly sensitive to the 31 amino acid βE peptide, but that patterns are very different for other peptides. Furthermore, the patterns of immunoreactivity of the antisera used in this study (Table 1) differ considerably from those used by Leboyer, et al. [23, Table 1, p. 1798]. The cross-reactivity profile and the very high level of β-LP peptide measured by antiserum for the C-terminal fragment suggests that perhaps their assay is primarily detecting the intact (or nearly intact) β-LP fragment, whereas our BEC-IR also detects more βE1-27 (75% cross-reactivity). The antibody for the fragment they labeled “N-terminal” is not highly cross-reactive with βE1-16 (11%) whereas ours cross-reacts 69%. Possibly the failure of the Leboyer group to detect elevated BEN in autistic patients is related to their assay being less immunoreactive to the N-terminal than our BEN-IR assay. However, it is not clear that our BEC-IR assay is sufficiently different from theirs to explain the differing C-terminal results. Their research has found markedly elevated BEC in 80% of autistic patients, many of which had SIB symptoms [4,23].
We used human antisera to assess macaque POMC fragments since there are notable genetic similarities between macaques and humans. The mu-opioid receptor coding region in rhesus is approximately 98% homologous to the corresponding human coding region [28]. Naltrexone antagonizes particularly mu-type opioid receptors [4]. The amino acid sequence for M. nemestrina βE1-31 is identical to that of H. sapiens except for one substitution in the last position, at the end of the C-terminal [29] that may possibly contribute to the contrasting results we found for BEC, but not for BEN.
In the two macaque species studied using the assays described in Table 1, there is clear evidence of an association between abnormal behavior and the N-terminal, but not the C-terminal, βE fragment. This is the first demonstration of a link between abnormal behavior and the N-terminal fragment (BEN) in a nonhuman primate, supporting the role of opioid action in that region of the POMC molecule. Previous human research has identified the N-terminal as a region of opioid activity. A study of human monocyte chemotactic activity of beta-endorphin and related peptides found that N-terminal-mediated effects were naloxone reversible, whereas the C-terminal effects were not; the authors concluded that the intact N-terminal is necessary for opioid-like effects [39].
In this study, C-terminal immunoreactivity (BEC) is related to a monkey's sex, but not its abnormal behavior. The trend toward significance of the sex factor in BET (Table 5) appears to be related to sex differences in the C-terminal, because the MANCOVA controlled for sex differences in abnormal behavior. It is possible that some human studies indicating opioid action in the C-terminal are confounded by uncontrolled sex differences, although this was not the case for Bouvard [4] in which five subjects were male and five were female. In our study, male macaques were more likely to have higher abnormal behavior rank scores than females. In general, male macaques are significantly more likely than females to exhibit SIB and other serious abnormal behaviors [31,32]. Among developmentally delayed (DD) humans, SIB is slightly more prevalent in males [38].
The species did not differ significantly in levels of abnormal behavior. This study revealed no statistically significant species differences in any of the peptides. Mf males had the highest levels of BET and Mn females, the lowest, consistent with Mf males having the highest average AbnRank scores and Mn females, the lowest (Table 3). The beta-endorphin (BET) levels of ∼12 pg/ml reported previously for female Mf [18,27] are intermediate between the 17.7 pg/ml BET we found for female Mf and the 8.5 pg/ml for female Mn (Table 3). McCubbin, et al. [27] measured βE-like immunoreactivity with a commercially available radioimmunoassay kit with <5% cross-reactivity with β-LP.
3.2 ACTH, cortisol, and dysregulation index
The GLM-MANCOVA found no significant relation between abnormal behavior and the ACTH and cortisol variables. These factors were weakly significant in Spearman correlations (Table 4), but the significance evaporated when MANCOVA took into account multiple factors simultaneously (Table 5). The uncontrolled interval between sedation and venipuncture, and variations in time of blood draw, may have contributed variability in ACTH and cortisol levels, which appear to be somewhat elevated. However, opioid levels can also be influenced by these factors, yet significant BET, BEC, and BEN effects were found. In macaques, both ACTH and cortisol vary with time of day, being higher in the morning [7]. The plasma cortisol values in the present study averaged 38 μg/dl, compared with baseline morning values of 27 μg/dl from M. mulatta [2,5] and ∼23 μg/dl from M. fascicularis [10]. In response to a stressor of mock capture, morning cortisol values for M. fascicularis rose from 30 μg/dl to 46 μg/dl [27]. ACTH values in the present study averaged 63 pg/mg, compared with morning values of 47-56 pg/ml for male M. mulatta [7] and afternoon values of 15-32 pg/ml for male M. mulatta [6]. In female macaques (M. mulatta, M. fascicularis, M. radiata), plasma ACTH and cortisol levels rose significantly after exposure to acute stressors (awake venipuncture, hand-capture, transport, telemetry jacket); values (average of morning and afternoon) were ∼40 pg/ml baseline and ∼67 pg/ml stressed for ACTH, and ∼27 μg/dl baseline and 50 μg/dl stressed for cortisol [8]. Cortisol levels can rise within minutes of a stressor such as sedation for blood draw. Further sampling (Bentson, in progress), in which the time of day and rapidity of blood draw are more tightly controlled, may clarify the significance of the dysregulation index, ACTH, and cortisol with respect to abnormal behavior in these macaque species.
3.3 Positive versus negative associations between beta-endorphin and behavior disorders
Our longtailed and pigtailed macaque subjects showed a positive relation between abnormal behavior and BET and BEN. Recent research on rhesus macaques (M. mulatta) found significantly reduced basal levels of βE-like immunoreactivity in blood plasma of monkeys with a veterinary record of SIB wounding [53]; the specific assay was not described. Their results for M. mulatta appear to be inconsistent with the present results for M. nemestrina and M. fascicularis, where βE-immunoreactivity (BET and BEN) are positively associated with overall rating of abnormal behavior. In our study, SIB subjects received the top 8 rankings because they were regarded as the most severe abnormal behavior cases. Because our sample included only eight SIB subjects and because the human literature defines SIB more broadly, we did not directly compare SIB versus non-SIB subjects.
These contrasting results may reflect species or genetic differences, or differences in assays, in severity or frequency of SIB, methods for quantifying abnormal behavior, or other factors. For example, Tiefenbacher, et al. [53] cite several studies supporting their findings in which autistic humans were reported to have significantly lower levels of beta-endorphin, yet the Leboyer group found elevated BEC-IR in most autistic patients [4,23]. SIB and other forms of abnormal behavior may represent a variety of etiologies [1,14,51,53], as might their relation to endogenous opioids. Tiefenbacher et al. [53] suggested that rhesus macaques with lower basal levels of βE may be more prone to engage in self-biting to increase low levels of endogenous opioids. SIB in their monkeys might have some parallels with alcoholism. The mu-opioid receptor plays a role in regulating the effects of alcohol, and alcohol consumption increases levels of βE, probably through increased release of POMC [58]. Compared to a low-risk group, humans at high risk for developing alcoholism, have lower levels of βE and increased plasma βE in response to small doses of ethanol [58]. Some macaques have an allele of the mu-opioid receptor gene that produces more than three times the affinity for βE, i.e., for a given level of βE, greater βE binding produces increased opioid effects in the body [28]. Rhesus macaques with this allele have lower plasma cortisol and higher rates of aggressive threat [28]. Possibly macaques with this allele are also more likely to self-bite. However, another study found SIB and externally directed aggression in male rhesus macaques to be unrelated [25].
The negative relation between SIB and βE reported for rhesus macaques [53] is in the opposite direction suggested by the present study of longtailed and pigtailed macaques, and that reported for humans. Opioid blockers generally are associated with reductions in abnormal behavior, although the type of behavior affected varies across studies. Although some studies have specifically linked stereotypy to endorphins [11], in other studies opioid antagonists have reduced both stereotypic and normal activities [21]. One speculation is that opioid antagonists reduce the persistence of behavior but not necessarily all stereotypic behavior [21]. Human patients with SIB and stereotypy showed decreased SIB but no systematic effects on stereotypy when administered naltrexone [41]. Subsequent research found SIB to be more effectively treated by naltrexone in patients with higher levels of βE [43,44]. Among developmentally delayed (DD) humans, those with SIB plus stereotypy had significantly higher morning plasma βE levels than DD patients without these behaviors. Those patients with SIB or stereotypy, but not both, had slightly higher levels of βE than those without either [40]. However, the combined patient groups had lower levels of βE than non-DD controls [40].
Some answers may lie in individual differences in opioid responses to stress. Stress is known to increase the level of POMC products, including βE [54]. A study of female M. fascicularis exposed to a stressor (person wearing monkey capture glove) found naloxone to produce significant increases in plasma cortisol and βE-like immunoreactivity compared with saline [27]. The monkeys with high heart-rate reactivity to stress had a very different response to naloxone than those with lower heart-rate reactivity, suggesting that opioids were less effective in inhibiting adrenocortical and circulatory responses in the former [27]. The less reactive naloxone-treated monkeys mounted a much greater increase in βE in response to stress than did the high heart-rate reactors. This suggested that “normal” monkey opioid systems effectively modulate responses to stress.
It may be that individuals with abnormally low levels of βE that are exposed to stressors -or are more reactive to stressors - need to engage in higher levels of stereotypy or SIB to mount an effective stress response. If performing SIB, noninjurious self-biting, or stereotypies increase βE, then βE should be positively correlated with abnormal behavior rank if other factors are equal. Because other factors usually are not equal, reported patterns may vary. As this study and some others have shown, the pattern of beta-endorphin response varies depending upon the fragments measured. Clearly, more research is warranted.
4. Conclusions
The significant positive correlation between beta-endorphin and abnormal behavior ratings in longtailed and pigtailed macaques is consistent with the hypothesis that abnormal behavior might be maintained by the sensations produced by endogenous opioid release. This could explain why such behaviors, once established, are difficult to treat. However, these results do not address hypotheses about origins of the abnormal behavior, nor whether the sensations proposed to be reinforcing are pleasurable (euphoric), pain-reducing (analgesic), or both.
The finding in the present study that intact beta-endorphin is positively correlated with AbnRank may reflect heightened stress responsiveness to sample collection and/or higher baseline levels of POMC products in monkeys that exhibit behavioral abnormalities. The positive correlation is consistent with two hypotheses regarding endorphins and abnormal behavior. Endorphins provide analgesia and euphoria. Monkeys with high endorphin levels may be less sensitive to physical discomfort that may result from self-biting. Alternatively, monkeys may engage in abnormal behaviors if doing so increases endorphin levels and produces euphoria.
The present results contrast with the negative correlation between beta-endorphin and SIB reported for rhesus macaques [53]. However, both studies confirm that an association exists between abnormal behavior and endogenous opioids in macaques. The present study, by examining three beta-endorphin fragments using sensitive assays, is the first to demonstrate a positive relation of the N-terminal fragment to abnormal behavior in a nonhuman primate. Another new finding is that, in these macaques, C-terminal variation is associated with a macaque's sex but not its level of abnormal behavior.
Acknowledgments
We thank the Research Support staff of WaNPRC for sample collection, Tom Burbacher for access to the methylmercury subjects, Rita Bellanca, Diella Koberstein, Kelly Heffernan, Maryam Salt, and Noelle Liberato for information contributing to the behavior ratings, and James Ha for genetics advice. This work was facilitated by grant P30 HD02274 from the National Institute of Child Health and Human Development. This research was supported by NIH RR00166 (CMC, GPS, KLB) and NICHD HD31571 (CS, ACD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- 2.Bentson KL, Capitanio JP, Mendoza SP. Cortisol responses to immobilization with Telazol or ketamine in baboons (Papio cynocephalus/anubis) and rhesus macaques (Macaca mulatta) J Med Primatol. 2003;32:148–60. doi: 10.1034/j.1600-0684.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 3.Bolles RC, Fanselow MS. Endorphins and behavior. Ann Rev Psychol. 1982;33:87–101. doi: 10.1146/annurev.ps.33.020182.000511. [DOI] [PubMed] [Google Scholar]
- 4.Bouvard MP, Leboyer M, Launay JM, Recasens C, Plumet MH, Waller-Perotte D, et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: a double-blind, placebo-controlled study. Psychiatry Research. 1995;58:191–201. doi: 10.1016/0165-1781(95)02601-r. [DOI] [PubMed] [Google Scholar]
- 5.Capitanio JP, Mendoza SP, Bentson KL. Personality characteristics and basal cortisol concentrations in adult male rhesus macaques (Macaca mulatta) Psychoneuroendo. 2004;29:1300–8. doi: 10.1016/j.psyneuen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): Evidence for temporal and situational consistency. Am J Primatol. 1998;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Carnes M, Kalin NH, Lent SJ, Barksdale CM, Brounfield MS. Pulsatile ACTH secretion: Variation with time of day and relationship to cortisol. Peptides. 1988;9:325–31. doi: 10.1016/0196-9781(88)90268-9. [DOI] [PubMed] [Google Scholar]
- 8.Clarke AS. ACTH and glucocorticoid responses under two conditions of stress in macaques. Am J Primatol. 1991;25:115–24. doi: 10.1002/ajp.1350250205. [DOI] [PubMed] [Google Scholar]
- 9.Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemp Top Lab Anim Sci. 1997;36:53–60. [PubMed] [Google Scholar]
- 10.Crockett CM, Bowers CL, Sackett GP, Bowden DM. Urinary cortisol responses of longtailed macaques to five cage sizes, tethering, sedation, and room change. Am J Primatol. 1993;30:55–74. doi: 10.1002/ajp.1350300105. [DOI] [PubMed] [Google Scholar]
- 11.Cronin GM, Wiepkema PR, van Ree JM. Endorphins implicated in stereotypies of tethered sows. Experientia. 1986;42:198–9. doi: 10.1007/BF01952467. [DOI] [PubMed] [Google Scholar]
- 12.Curin JM, Terzic J, Petkovic ZB, Zekan L, Terzic IM, Susnjara IM. Lower cortisol and higher ACTH levels in individuals with autism. J Autism Dev Disord. 2003;33:443–8. doi: 10.1023/a:1025019030121. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R. Behavioral, physiological and functional aspects of stereotyped behavior: a review and a re-interpretation. Journal of Animal Science. 1986;62:1776–86. doi: 10.2527/jas1986.6261776x. [DOI] [PubMed] [Google Scholar]
- 14.Garner JP. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR Journal. 2005;46:106–17. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert SG, Rice DC, Burbacher TM. Fixed interval/fixed ratio performance in adult monkeys exposed in utero to methylmercury. Neurotoxicol Teratol. 1996;18:539–46. doi: 10.1016/0892-0362(96)00081-5. [DOI] [PubMed] [Google Scholar]
- 16.Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Annals of the New York Academy of Science. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- 17.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendo. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 18.Kerdelhue B, Jones GS, Gordon K, Seltman H, Lenoir V, Parsadaniantz SM, et al. Activation of the hypothalamo-anterior pituitary corticotropin-releasing hormone, adrenocorticotropin hormone and beta-endorphin systems during the estradiol 17-beta-induced plasma LH surge in the ovariectomized monkey. Journal of Neuroscience Research. 1995;42:228–35. doi: 10.1002/jnr.490420210. [DOI] [PubMed] [Google Scholar]
- 19.Kidney CM, MacDonald JM, Angarano DW, Insalaco TA, Kempainnen RJ, Sartin JL. Amplification of proopiomelanocortin mRNA in canine skin: preliminary results. Veterinary Dermatology. 2004;15:389–91. doi: 10.1111/j.1365-3164.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 20.Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neuroscience and Biobehavioral Reviews. 2004;27:777–86. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Ladewig J, de Passillé AM, Rushen J, Schouten W, Terlouw EMC, von Borell E. Stress and the physiological correlates of stereotypic behaviour. In: Lawrence AB, Rushen J, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CAB International; Wallingford UK: 1993. pp. 97–118. [Google Scholar]
- 22.Lawrence AB, Rushen J, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. CAB International; Wallingford UK: 1993. p. 212. [Google Scholar]
- 23.Leboyer M, Bouvard MP, Recasens C, Philippe A, Guilloud-Bataille M, Bondoux D, et al. Difference between plasma N- and C-terminally directed beta-endorphin immunoreactivity in infantile autism. Am J Psychiatry. 1994;151:1797–801. doi: 10.1176/ajp.151.12.1797. [DOI] [PubMed] [Google Scholar]
- 24.Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Society of Biological Psychiatry. 1999;45:58–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 25.Lutz CK, Marinus LM, Chase WK, Meyer JS, Novak M. Self-injurious behavior in male rhesus macaques does not reflect externally directed aggression. Physiol Behav. 2003;78:33–9. doi: 10.1016/s0031-9384(02)00886-7. [DOI] [PubMed] [Google Scholar]
- 26.Marinus LM, Chase WK, Novak MA. Self-biting in rhesus macaques (Macaca mulatta) is preferentially directed to body areas associated with acupuncture analgesia. Am J Primatol. 2000;51:71–2. [Google Scholar]
- 27.McCubbin JA, Kaplan JR, Manuck SB, Adams MR. Opioidergic inhibition of circulatory and endocrine stress responses in cynomolgus monkeys: A preliminary study. Psychosom Med. 1993;55:23–8. doi: 10.1097/00006842-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Molecular Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Biotechnology Information NCBI Entrez Protein Database. 2007 http://www.ncbi.nlm.nih.gov/sites/entrez.
- 30.Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 31.Novak MA. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- 32.Novak MA, Crockett CM, Sackett GP. Self-injurious behavior in captive macaque monkeys. In: Schroeder S, Oster-Granite ML, Thompson T, editors. Self-Injurious Behavior: Gene-Brain-Behavior Relationships. APA Books; Washington, D. C.: 2002. pp. 151–61. [Google Scholar]
- 33.Patel PD, Sherman TG, Watson SJ. Characterization of pro-opioomelanocortin cDNA from the Old World monkey, Macaca nemestrina. DNA. 1988;7:627–35. doi: 10.1089/dna.1988.7.627. [DOI] [PubMed] [Google Scholar]
- 34.Rapp JT, Vollmer TR. Stereotypy I: A review of behavioral assessment and treatment. Research in Developmental Disabilities. 2005;26:527–47. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Rapp JT, Vollmer TR. Stereotypy II: A review of neurobiological interpretations and suggestions for an integration with behavioral methods. Research in Developmental Disabilities. 2005;26:548–64. doi: 10.1016/j.ridd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Ribka EP, Baker KC. Naltrexone (naltrexone hydrochloride) as a treatment to decrease incidence and severity of self-injurious behavior in rhesus macaques (Macaca mulatta) Am J Primatol. 2004;62:44. [Google Scholar]
- 37.Rodbard D, Hutt DM. Radioimmunoassays and Related Procedures in Medicine. Vol. 1. Atomic Energy Agency; Vienna: 1974. Statistical analysis of radioimmunoassays and immunoradiometric (labeled antibody) assays: A generalized, weighted, iterative, least-squares method for logistic curve fitting; pp. 165–72. [Google Scholar]
- 38.Rojahnn J, Esbensen AJ. Epidemiology of self-injurious behavior in mental retardation: a review. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious behavior: Gene-Brain-Behavior Relationships. American Psychological Association; Washington DC: 2002. pp. 41–77. [Google Scholar]
- 39.Sacerdote P, Panerai AE. Analysis of the beta-endorphin structure-related activity on human monocyte chemotaxis: Importance of the N- and C-terminal. Peptides. 1988;10:565–9. doi: 10.1016/0196-9781(89)90143-5. [DOI] [PubMed] [Google Scholar]
- 40.Sandman CA, Barron JL, Chicz-DeMet A, DeMet EM. Plasma B-endorphin levels in patients with self-injurious behavior and stereotypy. American Journal on Mental Retardation. 1990;95 [PubMed] [Google Scholar]
- 41.Sandman CA, Barron JL, Colman H. An orally administered opiate blocker, Naltrexone, attenuates self-injurious behavior. American Journal of Mental Retardation. 1990;95:93–102. [PubMed] [Google Scholar]
- 42.Sandman CA, Hetrick W, Taylor D, Marion S, Chicz-DeMet A. Uncoupling of proopiomelancortin (POMC) fragments is related to self-injury. Peptides. 2000;21:785–91. doi: 10.1016/s0196-9781(00)00209-6. [DOI] [PubMed] [Google Scholar]
- 43.Sandman CA, Hetrick WP, Taylor DV, Marion SD, Touchette P, Barron JL, et al. Long-term effects of Naltrexone on self-injurious behavior. American Journal on Mental Retardation. 2000;105:103–17. doi: 10.1352/0895-8017(2000)105<0103:LEONOS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Sandman CA, Touchette P. Opioids and the maintenance of self-injurious behavior. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious behavior: Gene-Brain-Behavior Relationships. American Psychological Association; Washington DC: 2002. pp. 191–204. [Google Scholar]
- 45.Sandman CA, Touchette P, Marion S, Lenjavi M, Chicz-Demet A. Disregulation of proopiomelanocortin and contagious maladaptive behavior. Regulatory Peptides. 2002;108:179–85. doi: 10.1016/s0167-0115(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious Behavior: Gene-Brain-Behavior Relationships. American Psychological Association; Washington, D. C.: 2002. p. 405. [Google Scholar]
- 47.Shulman LM, Sanchez-Ramoz JR, Weiner WJ. Defining features, clinical conditions, and theoretical constructs of stereotyped movements. In: Sprague RL, Newell KM, editors. Stereotyped Movements: Brain and Behavior Relationships. American Psychological Association; Washington, D. C.: 1996. pp. 17–34. [Google Scholar]
- 48.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocrine Reviews. 1988;9:159–79. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 49.Sprague RL, Newell KM, editors. Stereotyped Movements: Brain and Behavior Relationships. American Psychological Association; Washington, D. C.: 1996. p. 211. [Google Scholar]
- 50.Thompson T, Caruso M. Self-injury: knowing what we're looking for. In: Schroeder SR, Oster-Granite ML, Thompson T, editors. Self-Injurious behavior: Gene-Brain-Behavior Relationships. American Psychological Association; Washington DC: 2002. pp. 3–21. [Google Scholar]
- 51.Tiefenbacher S, Fahey MA, Rowlett JK, Meyer JS, Pouliot AL, Jones BM, et al. The efficacy of diazepam treatment for the management of acute wounding episodes in captive rhesus macaques. Comparative Medicine. 2005;55:387–92. [PubMed] [Google Scholar]
- 52.Tiefenbacher S, Novak MA, Jorgensen MJ, Meyer JS. Physiological correlates of self-injurious behavior in captive, socially reared rhesus monkeys. Psychoneuroendo. 2000;25:799–817. doi: 10.1016/s0306-4530(00)00027-5. [DOI] [PubMed] [Google Scholar]
- 53.Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. The physiology and neurochemistry of self-injurious behavior: a nonhuman primate model. Frontiers in Bioscience. 2005;10:1–11. doi: 10.2741/1500. [DOI] [PubMed] [Google Scholar]
- 54.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 55.U. S. Department of Agriculture Animal Welfare, Standards, Final Rule (Part 3, Subpart D: Specifications for the humane handling, care, treatment, and transportation of nonhuman primates) Fed Register. 1991;56:6495–505. [Google Scholar]
- 56.Velleman PF. Data Desk: The New Power of Statistical Vision. Data Description Inc.; Ithaca, NY: 1997. p. 358. [Google Scholar]
- 57.Yehuda R, Bierer LM, Schmeidler J, Aferiat DH, Breslau MA, Dolan S. Low cortisol and risk for PTSD in adult offspring of Holocaust survivors. Am J Psychiatry. 2000;157:1252–9. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- 58.Zalewska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26:701–5. doi: 10.1016/j.peptides.2004.11.010. [DOI] [PubMed] [Google Scholar]