Summary
By using a non-cancer and a cancer cell line originally from the same tissue (colon), coupled with testing lectins for cell binding and for their effects on these cell lines in culture, this study decribes a simple multiparameter approach that has revealed some interesting results that could be useful in drug development strategies.
Two human cell lines, CCL-220/Colo320DM (human colon cancer cells, tumorigenic in nude mice) and CRL-1459/CCD-18Co (non-malignant human colon cells) were tested for their ability to bind to agarose microbeads derivatized with two lectins, peanut agglutinin (Arachis hypogaea agglutinin, PNA) and Dolichos biflorus agglutinin (DBA), and the effects of these lectins were assessed in culture using the MTT assay.
Both cell lines bound to DBA-derivatized microbeads, and binding was inhibited by N-acetyl-D-galactosamine, but not by L-fucose. Neither cell line bound to PNA-derivatized microbeads. Despite the lack of lectin binding using the rapid microbead method, PNA was mitogenic in culture at some time points and its mitogenic effect displayed a reverse-dose response. This was also seen with effects of DBA on cells in culture.
While this is a simple study, the results were statistically highly significant and suggest that : (1) agents may not need to bind strongly to cells to exert biological effects, (2) cell line pairs derived from diseased and non-diseased tissue can provide useful comparative data on potential drug effects and (3) very low concentrations of potential drugs might be initially tested experimentally because reverse dose responses should be considered.
Keywords: Lectin derivatized microbeads, CCL-220/Colo320DM, CRL-1459/CCD-18Co, PNA, DBA, lectin-cell binding
Introduction
Our group has developed a multi-faceted approach that can be used in the development of more specific drugs. We have tested the ability of cells to bind to lectin-derivatized agarose beads using yeast (Saccharomyces cerevisiae) cells (Salbilla et al., 1999; Navarro et al., 2002) or sea urchin embryos (Strongylocentrotus purpuratus) (Latham et al., 1995a; 1995b; 1999) and recently we have examined the cell surface properties of human colon cancer (CCL-220, CCL-255), human lung cancer (HTB-171) and human non-cancer colon (CRL-1459) cells (Khurrum et al., 2002; Heinrich et al., 2005; Welty et al., 2006).
Few past studies have examined all three of the parameters that we examine in this model system: cell binding of the agent, toxicity of the agent and testing both cancer and non-cancer cells of the same tissue type (Valentiner et al., 2003; Heinrich et al., 2005). Our recent work in this area involved the use of lectins that are known to be toxic to cells (Heinrich et al., 2005). Using Phaseolus vulgaris agglutinin (PHA-L) and wheat germ agglutinin (WGA), we found that at some time periods and at some concentrations, these lectins were differentially toxic to the cancer cell line (Heinrich et al., 2005).
In Khurrum et al. (2002), we studied four cell types in a comprehensive analysis of binding to 51 types of derivatized beads, including 21 different lectin preparations. We showed that the four cell types tested - human colon non-malignant (CRL-1459), human colon malignant tumorigenic in nude mice (CCL-220), human lung cancer (HTB-171) and human colon cancer not tumorigenic in nude mice (CCL-255) - possessed different binding properties to this suite of derivatized beads. Also, in Welty et al. (2006), we showed exquisite correlation between the short term derivitized-bead binding test and the long term fluorescence labeling of lectin binding. Only in one case did fluorescence labeling, at such a low level that it was not detectable photographically, indicate very slight cell binding where the derivatized-bead approach did not.
In the study reported here, we examine two lectins that are reported to be largely non-toxic to cells - Dolichos biflorus agglutinin (DBA) and Arachis hypogaea /peanut agglutinin (PNA) (Ryder et al., 1992; 1994; Chen et al., 1994; Camby et al., 1997; Kiss et al., 1997; Jordinson et al., 1998; Ohba et al., 2003; Valentiner et al., 2003; Singh et al., 2006) - using a human colon cancer (CCL-220) and a human non-cancer colon CRL-1459) cell line, as in our previous studies (Khurrum et al., 2002; Heinrich et al., 2005; Welty et al., 2006). We did not want to use toxic lectins that kill cells quickly. Instead, to fully explore binding and longer term effects we use non-toxic lectins in this study. We first test the ability of the two lectins DBA and PNA to bind to both cell lines and then we examine the effects of the lectins in culture.
Material and Methods
Cells lines & culture
CCL-220/Colo320DM (human colon cancer cells, tumorigenic in nude mice; originally obtained from a 55 yr old Caucasian female) and CRL-1459/CCD-18Co (non-malignant human colon cells; originally obtained from a 2.5month old African American male) were purchased from American Type Culture Collection (ATCC) (Manassas, VA). Tumorigenicity has been tested by ATCC and is detailed on their website (www.atcc.org) and in Quinn et al., (1979) and Trainer et al., (1988).
Cells were cultured in 75cm2 closed Falcon flasks (Fisher Scientific, Tustin, CA) at 37°C with 5% CO2 with RPMI 1640 cell culture medium (Sigma Chemical Co. St. Louis, MO) which was supplemented with 10%, heat-inactivated, fetal bovine serum (Fisher) and 0.5ml of 45% D-glucose solution in total of 100ml RPMI 1640 medium. Cell lines were maintained without the use of antibiotics and any contaminated cultures were disposed of. Details of subculture of these cell lines are provided in Heinrich et al. (2005) and Welty et al. (2006).
Fixing cells for the lectin bead binding assay
Both cell lines were fixed by the following method. Cells were centrifuged at 113 × g for 3 minutes; the growth medium was discarded and the cells were resuspended in 1ml RPMI 1640 medium. The cells were washed 3 times with PBS (without CaCl2 and MgCl2) and centrifuged for 3 minutes, between each wash, before the addition of 1% formaldehyde in PBS (with CaCl2 and MgCl2) (Sigma). The cells were incubated with 1% formaldehyde in PBS at room temperature for 45 minutes for the completion of the fixation process. Fixed cells were washed 3 times in distilled water, via centrifugation, and resuspended in 1ml distilled water to be used within 3 days. Fixed cells have previously been demonstrated to display the same lectin binding properties as live cells (Navarro et al., 2002; Welty et al., 2006).
Lectins
Lectin-agarose beads for the lectin bead binding assay
Agarose beads were derivatized either with Arachis hypogaea (PNA) or Dolichos biflorus (DBA) lectins (Sigma). The positive control used, for both cell lines, was poly-L-lysine derivatized beads. The use of the positive control was to verify the binding capabilities of the cell lines used. All beads were washed 3 times by centrifugation with distilled water, kept at 4°C and used within 3 days.
Lyophilized powdered lectins for the effects of lectin on cell viability
PNA (L-0881, Sigma) and DBA (L-1201, EY Labs, San Mateo, CA) were reconstituted in sterile PBS (without CaCl2 and MgCl2) at a concentration of 1mg/ml. PBS (without CaCl2 and MgCl2) was used as the buffer for both cell lines as it had been in other studies using these same cell lines (Heinrich et al., 2005, Welty et al., 2006). Also, PBS was used in Khurrum et al. (2002) in studies using PNA and DBA lectins. Stock solutions and lectin dilutions were prepared in serum-free RPMI 1640 medium and stored at −20°C.
Lectin specific sugars
Monosaccharides used in the hapten inhibition protocol were as follows. L-fucose (F2252, Sigma), at 0.75M, was used as the non-haptenic sugar for both of the lectins. DBA haptenic sugar was N-acetyl-D-galactosamine (A2795, Sigma) at a concentration of 0.75M. According to our preliminary results, PNA showed no binding to either cell line; hence, we did not use a haptenic sugar for PNA.
Lectin bead binding assay
Slides were prepared in triplicate. Suspensions of either 40μl of distilled water, a haptenic sugar or non-haptenic sugar, both in distilled water were prepared. Four micrograms of lectin beads were added to the suspensions and mixed with a Birchwood toothpick for exactly one minute. This process of mixing allowed for the interaction between the lectin beads and the sugar in the experiments using sugars. Then 4μl of washed formaldehyde-fixed cells were added and the suspension was mixed for another minute. Positive and negative binding was observed by light microscopy at 100x or 200x total magnification. Binding was confirmed by agitating the suspension, observing if the cells were bound to the lectin beads or were simply touching them. All assays were conducted in distilled water as this has been shown to be a reliable medium for such studies (Roque et al., 1996; Navarro et al., 2002; Khurrum et al., 2002; Heinrich et al., 2005; Welty et al., 2006).
Photography
Photomicrographs of the lectin-bead interaction with the cells were taken using a Zeiss (Oberkochen, Germany) Axiolab microscope at a magnification of 100x to 1000x.
Cell viability determination
Optimal cell concentration, for the MTT assay, was previously determined to be 5 × 105 cells/ml (Heinrich et al., 2005). This concentration was chosen based on the cells being plated at different concentrations and monitoring their viability over time. While we did not perform direct S-phase analysis as the cell concentration used at initial plating was low enough that the cell number continued to increase throughout the entire time course of the experiments.
Effects of lectins on cell lines in culture
Multi-well plates
65μl of cells (1.0 × 106 cells/ml) were plated in a multi-well plate (Fisher) for 24 hours prior to the addition of 65μl lectin solution at final concentrations of 0.05, 0.25, 0.5, 5.0, 25.0, and 50.0 μg/ml for the 6 and 12-hour incubations and 0.5, 1.0, 10.0, 50.0, and 100.0μg/ml for the 24, 48, and 72 hour incubations in RPMI 1640 medium. The same quantity of cells and no lectins in RPMI 1640 medium (controls) were added to some wells. According to the established MTT assay protocol, experiments were carried out in 5% fetal bovine serum with RPMI 1640 medium (Heinrich et al., 2005).
MTT assay
Tetrazolium derivative reduction assay (Sigma) was used to determine cell viability after cell-lectin incubation. As living cells respire, their mitochondrial dehydrogenases reduce yellow MTT (3-[4, 5- dimethylthiazol-2-yl]-2, 5-biphenyl tetrazolium bromide) to yield purple crystals. The intensity of the color product formed, due to the formation of the formazon crystals, is directly proportional to the number of live cells in each sample. MTT cell count is similar to a direct cell count and it is an important method for studies of cell viability (Remmelink et al., 1999). All the MTT experiments were conducted under sterile conditions. Bacterial or mycoplasma contamination increases the MTT color product formed. If purple crystals formed in minutes after the addition of the MTT, the experiment was stopped and the assay discarded (Heinrich et al., 2005; Denecke et al., 1999).
MTT was reconstituted in sterile PBS (without CaCl2 and MgCl2) at a final concentration of 5mg/ml. MTT vials were wrapped in foil to keep the solution in the dark, as MTT is light sensitive and will decompose, and were stored at −20°C. In each well sample of the multi-well plates used, 10μl of MTT was added to the cells for 3-hour incubation at 37°C, 5% CO2. Purple formazon crystals were dissolved with 10% Triton X-100 plus 0.1 N HCl in anhydrous isopropanol. The dissolved purple solution was incubated for 3-hours at 37°C, 5% CO2 and absorbance was measured at 570nm, with a background absorbance of 690nm, using a spectrophotometer (Spectramax 190, Molecular Devices, Sunnyvale, CA). Background absorbance is the absorbance of the wells or any cell debris which may have been produced by the addition of the MTT solubilization solution. The spectrophotometer subtracted background absorbance from the absorbance values of each well. Due to the plating technique at the time each sample was taken, variation of absorbance was seen between each plate of the same experimental condition. Therefore, average absorbance was taken for each lectin concentration at each time period and the average of the control cells was expressed as 100% cell survival. Each concentration of each lectin was tested with six wells in parallel per CCL-220 assay. For the CRL-1459 cell line, due to limited cell quantities, three wells were tested in parallel per assay. Each assay was run three times.
Statistical analysis of data
Standard error of the mean (SEM) was used to evaluate variation of absorbance detected between the different plates. Fisher F-Test (one-way analysis of variance) was performed, using Microsoft Excel 2003, to compare the results of each cell line and each experimental condition with each other. Values of p<0.05 were considered significant.
Results
Lectin bead binding assay
Results of the lectin bead binding assay are summarized in Table 1. Both cell lines, CCL-220 and CRL-1459, displayed positive binding to the poly-L-lysine control beads. DBA-derivatized beads displayed positive binding, in distilled water and in the presence of the non-haptenic sugar 0.75M L-fucose, to CCL-220 colon cancer cells and CRL-1459 non-cancer colon cells. In the haptenic inhibition assays, DBA binding to CCL-220 and CRL-1459 cells was inhibited in the presence of 0.75M N-acetyl-D-galactosamine. PNA beads did not bind to either cell line in distilled water or in the presence of 0.75M L-fucose. Figure 1 shows representative photos of cell binding to beads (a positive binding result) and lack of binding (a negative binding result).
Table 1.
Summary of results of derivitized bead-cell binding studies
| Cell Line | Bead | Testing Conditions | # of positive trials/number of total trials |
|---|---|---|---|
| CCL-220 | Dolichos biflorus (DBA) | Distilled Water
0.2M GalNAc 0.5M GalNAc 0.75M GalNAc 0.75M L-fucose |
6/6 (100%)
6/6 (100%) 6/6 (100%) 0/6 (0%) 6/6 (100%) |
| Arachis hypogaea (PNA) | Distilled Water
0.75M L-fucose |
0/6 (0%)
0/6 (0%) |
|
| Poly-L-lysine | Distilled Water | 6/6 (100%) | |
| CRL-1459 | Dolichos biflorus (DBA) | Distilled Water
0.2M GalNAc 0.5M GalNAc 0.75M GalNAc 0.75M L-fucose |
6/6 (100%)
6/6 (100%) 6/6 (100%) 0/6 (0%) 6/6 (100%) |
| Arachis hypogaea (PNA) | Distilled Water
0.75M L-fucose |
0/6 (0%)
0/6 (0%) |
|
| Poly-L-lysine | Distilled Water | 6/6 (100%) |
GalNAc = N-acetyl-D-galactosamine
Figure 1. Cell binding, or lack of binding, to lectin derivatized bead(s).
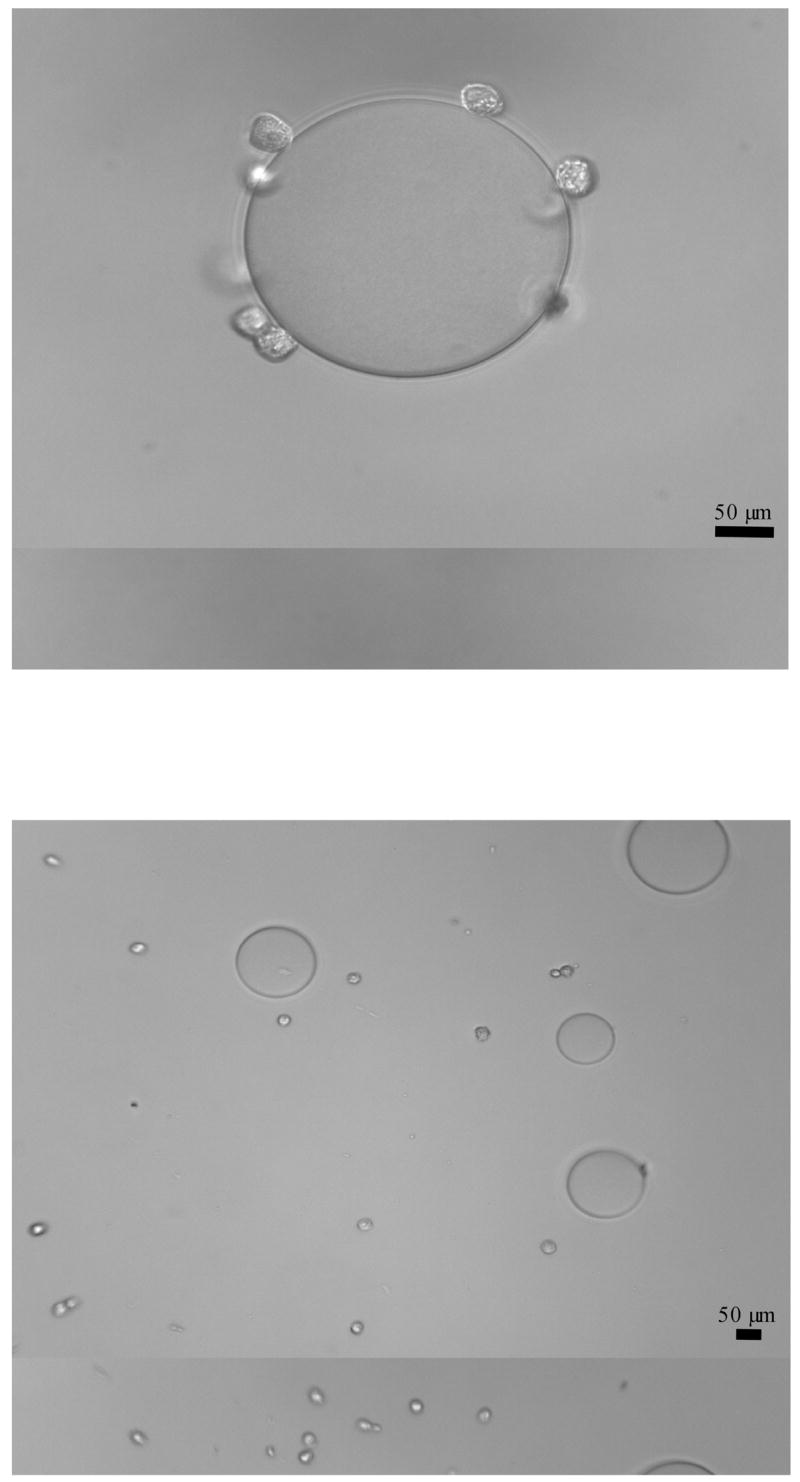
Top: Example of positive cell-bead binding. Fixed CCL-220 cells (small spheres) binding to a single DBA-derivatized agarose bead. Original magnification 400x.
Bottom: Example of negative cell-bead binding. Fixed CCL-220 cells (small spheres) not binding to DBA-derivatized agarose beads (large spheres) in the presence of 0.75M N-acetyl-D-galactosamine. Original magnification 200x.
Effects of lectins on cell lines in culture
PNA
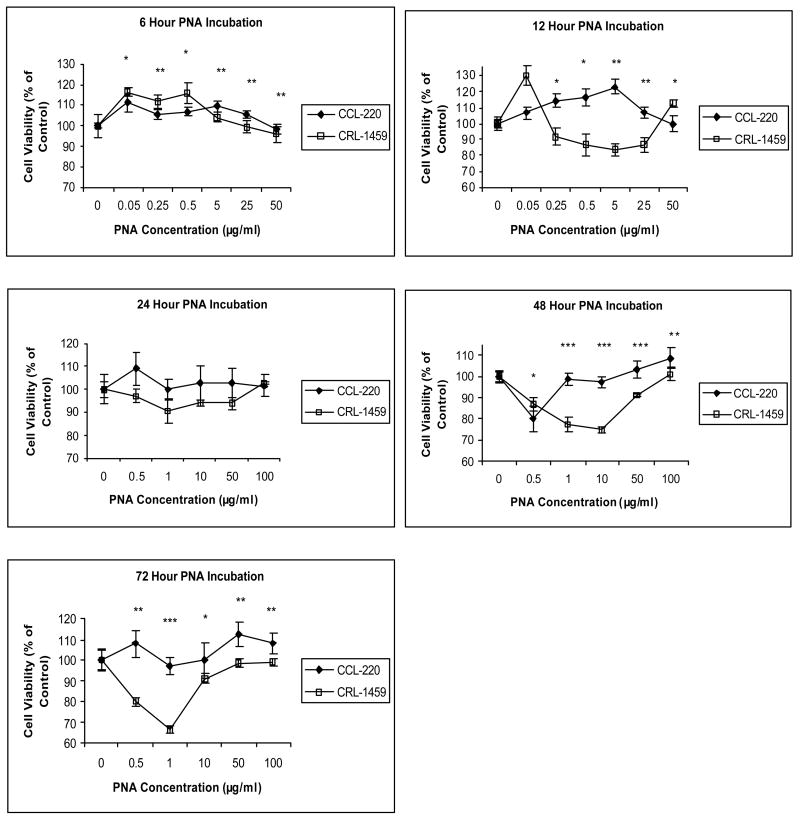
As shown in Figure 2, at 6 hrs incubation, PNA had a slight mitogenic effect on both cell lines. At 12 hrs, PNA was mitogenic for CCL-220 cells and only substantially at one concentration (0.05μg/ml) for CRL-1459 cells. At 24 hrs, there was little effect on either cell line, while at 48 hrs a reverse dose response was observed. For CRL-1459 cells at 0.05,1 and 10μg/ml, viability was reduced; however, viability then increased at 50 and 100μg/ml. CCL-220 cells also displayed a reverse dose response with reduced viability at 0.5μg/ml followed by increased viability at 1, 10. 50 and 100μg/ml. At 72 hrs, a very clear reverse dose response was observed for CRL-1459 cells, with substantially decreased viability at 0.5 and 1μg/ml followed by increased viability at 10, 50 and 100μg/ml. Little effect was observed with CCL-220 cells at 72 hrs
Figure 2. PNA effects on CCL-220 and CRL-1459 cells at 6, 12, 24, 48, 72 hours in culture.
*p<0.05, **p<0.01, ***p<0.001.
DBA
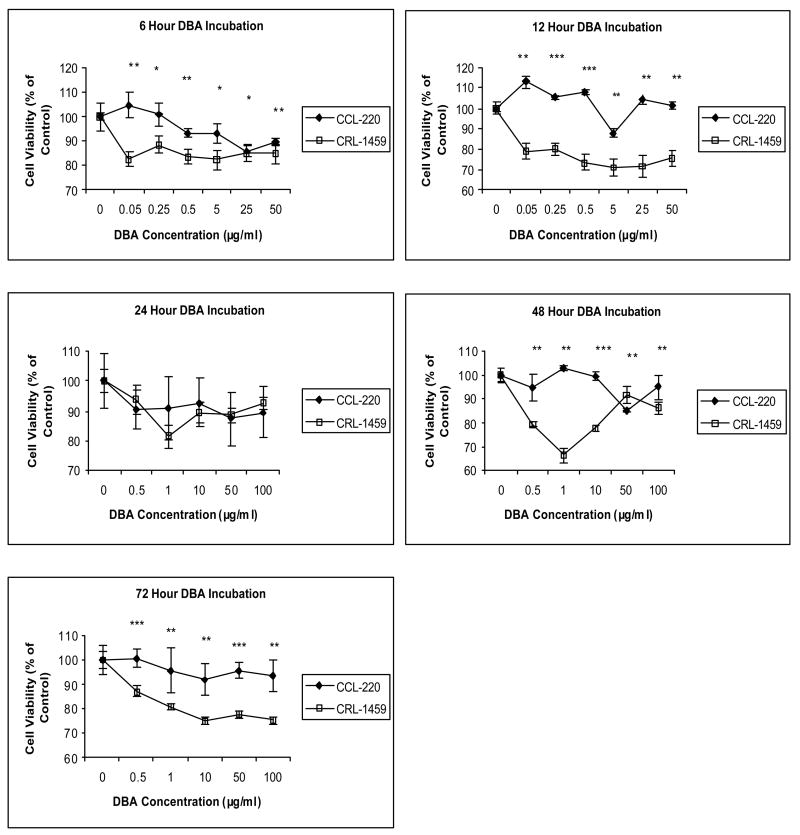
As shown in Figure 3, at 6 hrs DBA was slightly toxic to both cell lines. At 12 hrs, DBA was toxic to CRL-1459 and had little effect on CCL-220. At 24 hrs, slight toxicity was observed for both cell lines. At 48 hrs, a very clear reverse dose response was observed for CRL-1459 cells, with toxicity apparent at 0.5 and 1μg/ml and less toxicity at 10, 50 and 100μg/ml. Little effect was observed for CCL-220 cells. At 72, hrs DBA was toxic to CRL-1459 cells and had little effect on CCL-220 cells.
Figure 3. DBA effects on CCL-220 and CRL-1459 cells at 6, 12, 24, 48, 72 hours in culture.
*p<0.05, **p<0.01, ***p<0.001.
Discussion
In this study, we examined the binding of immobilized lectins, PNA and DBA, on two human colon cell lines, a non-cancer and a cancer cell line, and determined how the lectins affected these cells in culture. CCL-220 and CRL-1459 cells were fixed in 1% formaldehyde before testing their binding capabilities to PNA- and DBA-derivatized agarose beads. Both cell lines bound to immobilized DBA but not to immobilized PNA. In previous studies, having compared different cell preparation techniques, such as formaldehyde-fixed, Prefer-fixed and no fixation (live cells), we found that the tested fixatives did not alter lectin binding to cell surfaces (Navarro et al., 2002; Welty et al., 2006). In addition, results with the rapid bead binding assay correlated well with results using long and short term incubation with fluorescently labelled lectins (Latham et al., 1995a; 1995b; Welty et al., 2006).
The binding of lectins to cell surface ligands initiates the internalization of the lectins into the cell, sometimes leading to cell death via ribosomal inactivation or the initiation of cascade processes leading to apoptosis (Kim et al., 1993; Lorea et al., 1997; Gorelik et al., 2001; Gabor et al., 2001; 2004). Lectin binding to the cell glycocalyx can be inhibited by the addition of inhibitory sugars. The binding of specific sugars to the lectin binding sites inhibits lectin binding to the cells. Here, immobilized DBA, an N-acetyl-D-galactosamine binding lectin, was inhibited from binding to fixed cancer and non-cancerous cell lines in the presence of this monosaccharide. There was no inhibition of lectin-cell binding in the presence of L-fucose, a non-inhibitory sugar, indicating the binding of DBA to the cell surface was mediated through specific cell surface ligands.
A large number of studies with PNA indicate that this lectin acts in a cell type specific manner, often promoting proliferation and sometimes producing a toxic effect (Lee 1988; Caldero et al., 1989; McGarrity et al., 1991; Boland et al., 1992; Ryder et al., 1992; Chen et al., 1994; Kiss et al., 1997; Lorea et al., 1997; Jordinson et al., 1998; Yu et al., 2001; Singh et al., 2001; 2006; Ohba et al., 2003; Cai et al., 2005). Valentiner et al. (2003) in a model study showed that different lectins exerted different effects at specific concentrations on different breast cancer cell lines. Their results suggested that certain dietary lectins can inhibit growth of breast cancer cells. Here PNA sometimes induced cell proliferation or cell death depending on the time period course of the assay, the lectin concentrations and the cell line examined. The reverse-dose response effects found in the study reported here on these cell types has not been previously identified. Many investigators have used either the XTT assay kit (Valentiner et al., 2003) or the MTT assay kit (Kiss et al., 1997; Lorea et al., 1997; Cai et al., 2005; Heinrich et al., 2005) to determine cell proliferation or mitogenicity as we have in the current study.
As with PNA, studies have been reported on binding and the effects of DBA on various cell lines. DBA also acts in a cell type specific manner. It has usuallybeen reported not to be cytotoxic to cell lines tested (Chen et al., 1994; Gabor et al., 1998; Ohba et al., 2003). Here, DBA showed slightly toxic effects on both cell lines, particularly CRL-1459, at some time points and concentrations. As with PNA, an interesting reverse dose response was observed at 48 hrs with CRL-1459 cells.
In this study the non-cancer cells examined were more dramatically affected by the lectins than the cancer cells. While this study uses only two lectins and two cell lines, the statistically significant results indicate the potential usefulness of working with cancer cell and non-cancer cell line in parallel. This is important because many studies do not examine non-cancer counterparts when testing for the effects of potential anti-cancer drugs in culture. The findings here suggest that such testing should be used in these sorts of studies if the goal is to develop more specific anti-cancer drugs.
The reverse dose response results found here mainly with the CRL-1459 cell line are of major interest. Many studies begin with testing high concentrations of potential drugs on cells. If no effects are found with the high concentrations, the compound may be dropped from further testing. The results found in this study suggest that low concentrations might be tested initially. We show here that sometimes the effects are induced by low, not high, concentrations. Once again, this is a simple study that serves to alert investigators to the possibility of reverse dose responses. Other studies also suggest that a wide range of concentrations of agents should be initially tested for their effects on cells. Sano et al. (2000) found that migration of monocytes was best stimulated at 3μM galectin-3 while less stimulation occurred at 10μM or 0.01μM, while Kiss et al. (1997) found lectin induced growth stimulation and lectin induced growth inhibition at different concentrations. They also found greater effects of a lectin on growth stimulation at low concentrations with no effect at high concentrations.
Our results suggest that cells need not necessarily bind strongly to potential drugs for the drugs to affect them. The results with PNA on both cell types suggest that this is the case. While the bead assay that rapidly tests cell binding may not always detect very low levels of binding, previous studies show that results with the bead assay correlate almost identically with those using labeling over long term and short term with fluorescent labeled lectins (Latham et al., 1995a; 1995b; Welty et al., 2006). The current dogma assumes that molecules such as lectins must bind strongly to exposed cell surface components in order for internalization of the bound compound (eg lectin) to occur. However, plasma membrane invaginations can offer a mechanism for internalization of molecules by endocytosis without the need for binding of the molecules to accessible exposed membrane components (Nichols et al., 2001). Also, direct non-specific diffusion of proteins across membranes may offer another route of cellular entry of compounds such as lectins without extensive binding to specific cell surface components (Middlebrook et al., 1984). Gabor et al. (1998) found that some lectins very loosely or non-specifically bound to cells, if at all. As it is known that these lectins exert cell-type specific effects, it is possible that such loose associations can result in lectin internalization by mechanisms mentioned here and by others. In fact, internalization may not be necessarily required for lectins to exert their effects on some cell types. This issue is still unresolved and the results of this study suggest that the issue is more complex than previously believed.
In the development of anti-cancer drugs, lectins can be used directly as toxic agents such as has been done with mistletoe lectin (Fritz et al., 2004). They also can be used as carrier molecules that can be conjugated to chemotherapy drugs (Mody et al., 1995; Bies et al., 2004; Minko 2004). Currently, PNA is being used in this way (Minko 2004). Our results here show that at 12 hrs PNA was mitogenic to the cancer cell line (CCL-220) displaying maximum mitogenic activity at 5μg/ml. It was also mitogenic to the non-cancer colon cell line (CRL-1459) only at one concentration tested (0.05μg/ml). A potent drug could be coupled to a mitogenic lectin and the combination of mitogenicity and toxicity could synergistically potentiate the effects of the drug alone on cells. To our knowledge, although the issue of carrier molecules has been thoroughly explored (Mody et al., 1995; Bies et al., 2004; Minko 2004), the issue of mitogenic carriers has not been so well investigated.
In summary, while the study reported here only reports on two cell types and two lectins, the results illustrate a number of important issues: (1) comparing cell lines derived from diseased and non-diseased tissue can provide useful information on comparative drug effects; (2) agents may not need to strongly bind to cells to exert significant biological effects; and(3) initially testing very low concentrations of potential drugs is prudent because of the possibility of reverse dose responses. The types of issues raised in this paper could be helpful in the design of experiments and interpretation of the results in the area of drug development.
Acknowledgments
This work was supported by NIH NIGMS SCORE (S0648680), NIH MBRS RISE, NIH MARC, and the Joseph Drown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bies C, Lehr CM, Woodley JF. Lectin-mediated drug targeting: history and applications. Adv Drug Delivery Rev. 2004;56:425–35. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Boland CR, Martin MA, Goldstein IJ. Lectin reactivities as intermediate biomarkers in premalignant colorectal epithelium. J Cellular Biochem. 1992;Supplement 16G:103–09. doi: 10.1002/jcb.240501119. [DOI] [PubMed] [Google Scholar]
- Cai Q, Zhang ZR. Lectin-mediated cytotoxicity and specificity of 5- fluorouracil conjugated with peanut agglutinin (5-Fu-PNA) in vitro. J Drug Targeting. 2005;13:251–57. doi: 10.1080/10611860500138505. [DOI] [PubMed] [Google Scholar]
- Caldero J, Campo E, Ascaso C, Ramos J, Panades MJ, Rene JM. Regional distribution of glycoconjugates in normal, transitional and neoplastic human colonic mucosa. Virchows Arch A Pathol Anat Histopathol. 1989;415:347–56. doi: 10.1007/BF00718637. [DOI] [PubMed] [Google Scholar]
- Camby I, Salmon I, De Decker R, Pasteels JL, Brotchi J, Danguy A, Kiss R. Lectin histochemistry of astrocytic tumors and in vitro characterization of lectin-induced modifications on the proliferation of the SW1008, U373 and U87 human astrocytic cell lines. J Neuro-Oncology. 1997;34:111–122. doi: 10.1023/a:1005783321916. [DOI] [PubMed] [Google Scholar]
- Chen YF, Boland CR, Kraus ER, Goldstein IJ. The lectin Griffonia simplicifolia I-A4 (GS I-A4) specifically recognizes terminal a-linked N-acetylgalactosaminyl groups and is cytotoxic to the human colon cancer cell lines LS174t and SW1116. Int J Cancer. 1994;57:561–67. doi: 10.1002/ijc.2910570420. [DOI] [PubMed] [Google Scholar]
- Denecke J, Becker K, Jurgens H, Gross R, Wolff JEA. Falsification of tetrazolium dye (MTT) based cytotoxicity assay results due to mycoplasma contamination of cell cultures. Anticancer Res. 1999;19:1245–48. [PubMed] [Google Scholar]
- Fritz P, Dippon J, Kierschke T, Siegle I, Mohring A, Moisa A, Murdter TE. Impact of mistletoe lectin binding in breast cancer. Anticancer Res. 2004;24:1187–92. [PubMed] [Google Scholar]
- Gabor F, Stangly M, Wirth M. Lectin-mediated bioadhesion: binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. J Controlled Rel. 1998;55:131–42. doi: 10.1016/s0168-3659(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Gabor F, Klausegger U, Wirth M. The interaction between wheat germ agglutinin and other plant lectins with prostate cancer cells Du-145. Int J Pharm. 2001;221:35–47. doi: 10.1016/s0378-5173(01)00650-0. [DOI] [PubMed] [Google Scholar]
- Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv Drug Delivery Rev. 2004;56:459–80. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer Metastasis Rev. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- Heinrich EL, Welty LAY, Banner LR, Oppenheimer SB. Direct targeting of cancer cells: A multiparameter approach. Acta Histochem. 2005;107:335–44. doi: 10.1016/j.acthis.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordinson M, El-Hariry ID, Calnan D, Calam J, Pignatelli M. Vicia faba agglutinin, the lectin present in broad beans, stimulates differentiation of undifferentiated colon cancer cells. Gut. 1998;44:709–14. doi: 10.1136/gut.44.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurrum MR, Weerasinghe GR, Soriano ES, Riman R, Badali O, Gipson S, Medina J, Alfaro J, Navarro VM, Harieg CB, Ngo L, Sakhakorn T, Kirszenbaum L, Khatibi D, Abedi K, Barajas M, Zem GC, Kirszenbaum A, Razi A, Oppenheimer SB. Analysis of surface properties of human cancer cells using derivatized beads. Acta Histochem. 2002;104:217–23. doi: 10.1078/0065-1281-00656. [DOI] [PubMed] [Google Scholar]
- Kim M, Rao MV, Tweardy DJ, Prakash M, Galili U, Gorelik E. Lectin-induced apoptosis of tumour cells. Glycob. 1993;3:447–53. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- Kiss R, Camby I, Duckworth C, De Decker R, Salmon I, Pasteels J-L, Danguy A, Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, concanavalin A., wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997;40:253–61. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham VH, Ducut JL, Rostamiani K, Chun HH, Lopez ME, Herrera S, Oppenheimer SB. A rapid lectin receptor binding assay: comparative evaluation of sea urchin embryo cell surface lectin receptors. Acta Histochem. 1995a;97:89–97. doi: 10.1016/S0065-1281(11)80209-6. [DOI] [PubMed] [Google Scholar]
- Latham VH, Herrera S, Rostamiouni K, Chun H, Oppenheimer SB. Rapid identification of lectin receptors and their possible function on sea urchin cell systems. Acta Histochem. 1995b;97:373–82. doi: 10.1016/S0065-1281(11)80062-0. [DOI] [PubMed] [Google Scholar]
- Latham VH, Oppenheimer SB. A simple image analysis method for evaluating cell binding to derivatized beads. Acta Histochem. 1999;101:263–70. doi: 10.1016/S0065-1281(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Lee YS. Lectin expression in neoplastic and non-neoplastic lesions of the rectum. Pathology. 1988;20:157–65. doi: 10.3109/00313028809066627. [DOI] [PubMed] [Google Scholar]
- Lorea P, Goldschmidt D, Darro F, Salmon I, Bovin N, Gabius HJ, Kiss R, Danguy A. In vitro characterization of lectin-induced alterations on the proliferative activity of three human melanoma cell lines. Melanoma Res. 1997;7:353–63. doi: 10.1097/00008390-199710000-00001. [DOI] [PubMed] [Google Scholar]
- McGarrity TJ, Peiffer LP, Colony PC. Alterations in lectin binding in the proximal and distal colon of Sprague-Dawley rats with 1,2 dimethylhydrazine administration. Exp Pathol. 1991;41:175–83. doi: 10.1016/s0232-1513(11)80085-x. [DOI] [PubMed] [Google Scholar]
- Middlebrook JL, Dorland RB. Bacterial toxins: cellular mechanisms of action. Microbiological Rev. 1984;48:199–21. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Adv Drug Delivery Rev. 2004;56:491–09. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Mody R, Joshi S, Chaney W. Use of lectins as diagnostic and therapeutic tools for cancer. J Pharm Tox Methods. 1995;33:1–10. doi: 10.1016/1056-8719(94)00052-6. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Walker SL, Badali O, Abundis MI, Ngo LL, Weerasinghe G, Barajas M, Zem G, Oppenheimer SB. Analysis of surface properties of fixed and live cells using derivatized agarose beads. Acta Histochem. 2002;104:99–06. doi: 10.1078/0065-1281-00617. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends cell biol. 2001;11:406–12. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- Ohba H, Bakalova R. Relationships between the degree of binding, cytotoxicity and cytoagglutinating activity of plant-derived agglutinins in normal lymphocytes and cultured leukemic cell lines. Cancer Chemo and Pharm. 2003;51:451–58. doi: 10.1007/s00280-003-0607-y. [DOI] [PubMed] [Google Scholar]
- Quinn LA, Moore GE, Morgan RT, Woods LK. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979;39:4914–4924. [PubMed] [Google Scholar]
- Remmelink M, Darro F, Decaestecker C, De Decker R, Bovin NV, Gebhart M, Kaltner H, Gabius HJ, Kiss R, Salmon I, Danguy A. In vitro influence of lectins and neoglycoconjugates on the growth of three human sarcoma cell lines. J Cancer Res Clin Oncol. 1999;125:275–85. doi: 10.1007/s004320050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque RL, Herrera S, Yeh TJ, Philip J, Borisavljevic TL, Brunick L, Miles A, Haritunians T, Addy C, Bada RA, Vaghefi H, Matsumoto SS, Piccionelli GA, Rodriguez L, Oppenheimer SB. Cell adhesion mechanisms: modeling using derivatized beads and sea urchin cell sytems. Acta Histochem. 1996;98:441–51. doi: 10.1016/s0065-1281(96)80011-0. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Smith JA, Rhodes JM. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J Natl Cancer Inst. 1992;84:1410–16. doi: 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Parker N, Ecclestone D, Haqqani MT, Rhodes JM. Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology. 1994;106:117–124. doi: 10.1016/s0016-5085(94)94775-9. [DOI] [PubMed] [Google Scholar]
- Salbilla BA, Vaghefi H, Chhabra P, Hall G, Brown D, Sadoughi F, Francisco E, Attas L, Walker SL, Nguyen BN, Oppenheimer SB. Analysis of cell surface properties using derivatized agarose beads. Acta Histochem. 1999;101:271–79. doi: 10.1016/s0065-1281(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara I, Yamanaka T, Hirashima M, Liu FT. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J Immunol. 2000;165:2156–64. doi: 10.4049/jimmunol.165.4.2156. [DOI] [PubMed] [Google Scholar]
- Singh R, Campbell BJ, Yu LG, Fernig DG, Milton JD, Goodlad RA, FitzGerald AJ, Rhodes JM. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycob. 2001;11:587–92. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- Singh R, Subramanian S, Rhodes JM, Campbell BJ. Peanut lectin stimulates proliferation of colon cancer cells by interaction with glycosylated CD44v6 isoforms and consequential activation of c-Met and MAPK: functional implications for disease-associated glycosylation changes. Glycob. 2006;16:594–01. doi: 10.1093/glycob/cwj108. [DOI] [PubMed] [Google Scholar]
- Trainer DL, Kline T, McCabe FL, Faucette LF, Field J, Chaikin M, Anzano M, Rieman D, Hoffstein S, Li DJ, et al. Biological characterization and oncogene expression in human colorectal carcinoma cell lines. Int J Cancer. 1988;41( 2):287–96. doi: 10.1002/ijc.2910410221. [DOI] [PubMed] [Google Scholar]
- Valentiner U, Fabian S, Schumacher U, Leathem AJ. The influence of dietary lectins on the cell proliferation of human breast cancer cell lines in vitro. Anticancer Res. 2003;23:1197–1206. [PubMed] [Google Scholar]
- Welty LAY, Heinrich EL, Garcia K, Banner LR, Summers ML, Baresi L, Metzenberg S, Coyle-Thompson C, Oppenheimer SB. Analysis of unconventional approaches for the rapid detection of surface lectin binding ligands on human cell lines. Acta Histochem. 2005;107:411–20. doi: 10.1016/j.acthis.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LG, Milton JD, Fernig DG, Rhodes JM. Opposite effects on human colon cancer cell proliferation of two dietary Thomsen-Friedenreich antigen-binding lectins. J Cell Physiol. 2001;186:282–87. doi: 10.1002/1097-4652(200102)186:2<282::AID-JCP1028>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]