Abstract
Objective
The study was conducted to evaluate the effect of Carraguard vaginal gel containing 0.75 mg of levonorgestrel (CARRA/LNG gel) administered in a single dose at different stages of follicle development, over subsequent follicle rupture and hormonal levels.
Method
Randomized, blinded, cross-over study comparing the effects of a single administration of CARRA/LNG gel or Carraguard (CARRA) gel. Twenty-four healthy women were enrolled in two centers. The gels were administered when the follicle had reached diameters of 12-14, 15-17 and ≥18 mm in 8 women each. Volunteers were followed for one treatment, one washout cycle and a second treatment cycle. Follicle rupture or non-rupture was assessed by transvaginal ultrasound. LH, estradiol and progesterone levels were measured daily for the 5 days following treatment, and 3 times per week until menses.
Results
No follicular rupture within the 5-day period following administration was observed in 74% and 30% of the CARRA/LNG and CARRA gel treatment cycles, respectively, while ovulation was documented in 4% and 61%, respectively. The overall proportion of cycles with lack of follicular rupture or ovulatory dysfunction (follicle rupture preceded by an inadequate LH surge) was 96% for CARRA/LNG and 39% in the CARRA gel cycles.
Conclusion
Single vaginal administration of 0.75 mg levonorgestrel in CARRA gel in the late follicular phase, is effective for interfering with the ovulatory process.
Keywords: microbicides, emergency contraception, levonorgestrel, vaginal administration, ovarian function
1. Introduction
Healthy and satisfactory sexuality is a fundamental component of life fulfillment; however, sexual activity carries the risk of unintended pregnancy and the transmission of infections. Male and female condoms prevent both, but their contraceptive effectiveness is relatively low and their use is affected by male acceptance of either method. The human immunodeficiency virus (HIV) pandemic makes urgent the need to develop female controlled protection methods and much effort and funding has been allocated for the development of microbicides designed to prevent HIV transmission. However, many women also need protection against unintended pregnancy, and therefore there is demand for the development of dual protection microbicides
One non-contraceptive vaginal microbicide under intensive investigation at the Population Council for the prevention of HIV and sexually transmitted infections (STIs) in women is Carraguard (carrageenan PDR98-15: CARRA). Carrageenans have shown antiviral and antibacterial properties against HIV, herpes simplex virus-type 2 (HSV-2) and Neisseria gonorrhoeae [1-6]. The safety of vaginal administration has also been confirmed by laboratory and Phase 1 and 2 clinical studies [5, 7-9]. Furthermore, a large Phase III effectiveness trial is currently underway in South Africa.
More recently, the use of CARRA as a vehicle for vaginal delivery of a contraceptive steroid has been proposed. The Population Council’s Center for Biomedical Research developed a homogeneous suspension of the synthetic progestin levonorgestrel (LNG) in CARRA gel, CARRA/LNG, which allows the diffusion of the steroid out of the gel at a slow rate. One of the possible uses of this gel would be a pre-coital emergency contraceptive, for application before occasional intercourse. A recent pharmacokinetic study with CARRA/LNG containing 1.5 or 0.75 mg of levonorgestrel in 4 mL of gel, confirmed the vaginal absorption of this steroid [10]. LNG serum levels in the range known to interfere with the ovulatory process were achieved, thereby confirming the potential of CARRA/LNG gel as a new candidate product that could provide dual protection, both for prevention of pregnancy as well as prevention of STI.
We, therefore, proposed to evaluate whether single vaginal administration of CARRA/LNG gel applied prior to intercourse would interfere with the ovulatory process when administered in the fertile period of the cycle in a way similar to that already observed with the oral administration of LNG as emergency contraception.
This paper presents the effect of a single administration of CARRA/LNG 0.75 mg/4 mL or CARRA gel 4 mL, when given at different stages of follicular development, on subsequent follicular rupture and hormonal levels.
2. Materials and methods
The study was conducted at ICMER in Santiago, Chile, and PROFAMILIA in Santo Domingo, Dominican Republic. Approval was granted by the Ethics Committee of each center and by the Institutional Review Board of the Population Council (NY).
A total of 24 healthy women were enrolled, 12 at each center, after providing their informed consent. They were 21 to 40 years old, with regular menstrual cycles, protected from pregnancy by tubal ligation, non-breastfeeding, a body mass index <30 and had no contraindications to the use of oral contraceptives.
2.1. Study drug
CARRA/LNG formulation is a suspension solution combining Carraguard and levonorgestrel. It was manufactured at the Population Council’s Center for Biomedical Research in the laboratory of David M. Phillips (New York, NY). Carraguard contains purified water, 3% PDR98-15 carrageenan, and ρ-hydroxybenzoic methyl ester (methyl paraben) as a preservative. Hydrochloric acid and phosphate buffered saline (PBS) are used to adjust the pH to 7 and raise tonicity. The synthetic progestin levonorgestrel [D(-)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one] was added as micronized crystals and mixed.
2.2. Study design
This was a prospective, randomized, double-blind, cross-over, two-center study to evaluate the effects of single vaginal administration of CARRA/LNG gel delivering 0.75 mg of LNG in 4 mL gel, as compared to 4 mL CARRA gel alone (placebo). Subjects were randomized into three groups of 8 women each, based on the follicle diameter of the leading follicle at the time of treatment as follows: Group 1: 12-14 mm; Group 2: 15-17 mm and Group 3: > 18 mm. The order in which they received either CARRA/LNG gel delivering 0.75 mg of LNG or CARRA gel was also randomized. Each subject was followed for three cycles: first a placebo or drug treated cycle (CARRA gel or CARRA/LNG gel), then a resting washout cycle, and a final placebo or drug-treated cycle (CARRA gel or CARRA/LNG gel).
In both treated cycles, the same follow-up procedures were performed. Transvaginal ultrasonography (TVU) was used to assess the mean diameter (mean of longitudinal diameter and transverse diameter) of the largest (leading) follicle and the occurrence of follicular rupture. Starting on day 8 of the first cycle, TVU was done three times per week, until the pre-assigned follicular diameter for gel administration was approached. From then on, daily measurements were taken until the pre-assigned value set for the follicular diameter randomization scheme was met. When the pre-assigned follicular diameter was reached, each subject received a single intra-vaginal administration of either CARRA/LNG gel or CARRA gel. To limit dosing variability, the investigator administered the full volume of gel intravaginally, and subjects were asked to remain recumbent for 10 min.
For the following 5 consecutive days, daily TVU was performed. Thus, a total of 6 daily TVU were done, including the one done immediately before administering the vaginal gel. These 6 TVU encompass, from the first to the last, 5 periods of 24 h. This interval will be referred to as the 5-day period. From then on, if follicular rupture had not occurred, TVU were done three times per week until menses. TVU was performed with a real-time scanner, Shimadzu SDU-400 using a 5.0-MHz vaginal transducer, at Santo Domingo, and using a Medison Co. Ltd. SA 6000 ultrasound system, 7.5-MHz vaginal transducer, in Santiago.
A blood sample was taken immediately before starting treatment when the pre-assigned follicular diameter was reached. Daily sampling was continued in each of the ensuing 5 days and then three times per week until menses. Luteinizing hormone (LH), estradiol (E2) and progesterone (P) were measured in all samples. LNG was measured before gel administration and for the five consecutive days.
All subjects kept a record of occurrence and severity of adverse events, medications and bleeding data during the study using a specially designed diary. This diary was checked at each visit.
2.3. Hormone measurements
LNG in serum was measured at the Center for Biomedical Research of the Population Council, NY, by a conventional radioimmunoassay (RIA) method using a commercial kit (Immunometrics Ltd., London, United Kingdom). All samples from a subject were analyzed in the same assay run. The method is an extraction RIA and utilizes tritium-labeled LNG and the specific anti-LNG polyclonal antibody. The sensitivity of the assay was found to be 150 pmol/L (47 pg/mL). The assay is controlled through the use of three internal quality control specimens (low, medium and high with the LNG concentrations at 666, 1000, and 7000 pmol/L, respectively) in every assay. The intra- and inter-assay coefficients of variations were less than 10% in the LNG assays for the three controls used.
LH, E2 and P were measured locally at each clinic. In Chile, LH concentrations were measured by an enzyme-linked immunoassay (Immunometrics, Ltd; UK), with a sensitivity of 0.5 U/L, while E2 and P concentrations were measured by RIA (DPC, Diagnostic Products Corporation, Los Angeles, CA, USA) with a sensitivity of 21 pmol/L and 0.2 nmol/L, respectively. The inter-assay coefficients of variation were 6.2-8.9% for LH, 5.9-6.3% for progesterone and 6.8-9.3% for estradiol.
In the Dominican Republic, LH, P and E2 were measured by an electrochemiluminiscence immunoassay (ECLIA), in the Roche Elecsys 2010 immunoassay analyzer (Hitachi Corporation, Tokyo, Japan), with a sensitivity 0.10 U/L, 18 pmol/L and 0.1 nmol/L, respectively. The inter-assay coefficients of variation were 1.9-5.2 % for LH, 3.7-5.5% for P and 2.3-6.2% for E2, respectively.
2.4. Data analysis
The intervention variables under study were drug or placebo and timing of treatment. The response variables were the occurrence and timing of follicular rupture, LH and ovarian hormone levels in serum, LNG serum levels, bleeding and the incidence and severity of adverse events.
The following end-points and definitions were used for data analysis:
-
-
Length of the cycle: The number of days from the first day of menses until the day prior to the next menstrual-like bleeding, both inclusive.
-
-
Follicular rupture: Abrupt disappearance or a reduction in size, of at least 50% of the echo-image.
-
-
Ovulation: Follicular rupture preceded 24-48 h by a normal gonadotrophin surge and followed by a luteal phase, i.e., serum P concentration over 12 nmol/L, in at least 2 samples taken during the luteal phase.
-
-
Ovulatory dysfunction: Follicular rupture not preceeded by an LH peak or preceded by a blunted LH peak (< 21 IU/L), or not followed by elevation of serum P over 12 nmol/L.
-
-
Persistent follicle: A follicle developing beyond 15 mm and persisting for at least one week, without rupture, and without increase in P levels.
-
-
Luteinized unruptured follicle: Persistent echo-image of a follicle, associated with increased serum P levels.
-
-
Follicular atresia: no further growth or reduction in size of the dominant follicle.
The effect of treatment on the endocrine profile and the occurrence and timing of follicular rupture was more intensively investigated in the 5-day period commencing on the day of treatment, on the basis that following coitus, spermatozoa do not remain fertile beyond that period [11]. Therefore, if follicular rupture does not occur within this period, there would be no probability of conception had intercourse taken place. The proportion of cycles in which no follicular rupture was observed within the 5-day period, as well as the proportion of cycles with ovulatory dysfunction was compared between CARRA/LNG and CARRA, stratified by timing of gel administration.
Among cycles with follicular rupture documented within the 5-day period, mean LH levels were compared between cycles with ovulatory dysfunction and those classified as normal ovulation. Since the LH peak was not always evident, LH levels were tabulated and plotted in relation to the day of follicular rupture (Day 0).
Each volunteer recorded all adverse events she had in the following five days after gel application. The number of women reporting adverse events during the CARRA/LNG gel and CARRA gel cycles was compared.
All statistical analyses were performed using Graph Pad Prism software, version 3.02. Statistical tests used are indicated in the text, tables and figures as appropriate.
3. Results
Twenty-three of the 24 women enrolled completed the study as designed. One subject in the 15-17 mm follicular diameter group, ovulated before reaching the assigned diameter in two consecutive cycles, and was therefore discontinued from the study. As a result, we have data for 23 women each with the CARRA/LNG gel and CARRA. Twelve subjects completed the study in Santo Domingo and 11 in Santiago. The results of the study were similar for both centers, and therefore, were pooled together for the analysis. The mean body weight and body mass index was 59.5 ± 10.1 kg and 24.5 ± 3.3, respectively, and the mean age was 37.1 ± 3.0.
The mean follicular diameter and E2 levels at the time of treatment administration were similar for the two gels within each assigned follicular diameter group (Table 1).
Table 1.
Mean follicular diameter (mm) and E2 levels (pmol/L) at the time of treatment
| Assigned follicular diameter | n | Carra/LNG (0.75 mg/4 mL) | Carra 4 mL | ||
|---|---|---|---|---|---|
| Follicular diameter | E2 | Follicular diameter | E2 | ||
| 12-14 mm | 8 | 12.6 ± 0.7 | 329 ± 149 | 12.7 ± 0.5 | 268 ± 85 |
| 15-17 mm | 7 | 15.5 ± 0.6 | 719 ± 453 | 15.3 ± 0.5 | 645 ± 420 |
| ≥18 mm | 8 | 18.2 ± 0.3 | 702 ± 310 | 18.4 ± 0.7 | 789 ± 295 |
3.1. Lack of follicular rupture within the 5-day period and ovulatory dysfunction
No follicular rupture was observed in 74% of the CARRA/LNG cycles, and in 30% of the CARRA cycles. (p=0.0039). Inversely, ovulation was documented in 61% of the CARRA gel cycles, while it was only observed in 1 of the 23 CARRA/LNG cycles (4%) (Table 2). The percentage of LNG-treated cycles without follicular rupture was inversely proportional to the size of the leading follicle at the time of treatment (Fig. 1).
Table 2.
Distribution of cycles according to follicular outcome within the 5-day period following administration of CARRA/LNG or CARRA gel
| Classification | CARRA/LNG | CARRA | ||
|---|---|---|---|---|
| n=23 | n=23 | |||
| n | % | n | % | |
| Interference with ovulatory process | 22* | 96 | 9 | 39 |
| No follicular rupture within the 5-day period | 17** | 74 | 7 | 30 |
| Ovulatory dysfunction | 5 | 22 | 2 | 9 |
| Ovulation | 1* | 4 | 14 | 61 |
p=0.0008,
p=0.0039 versus CARRA;
McNemar chi-square test.
Fig. 1.
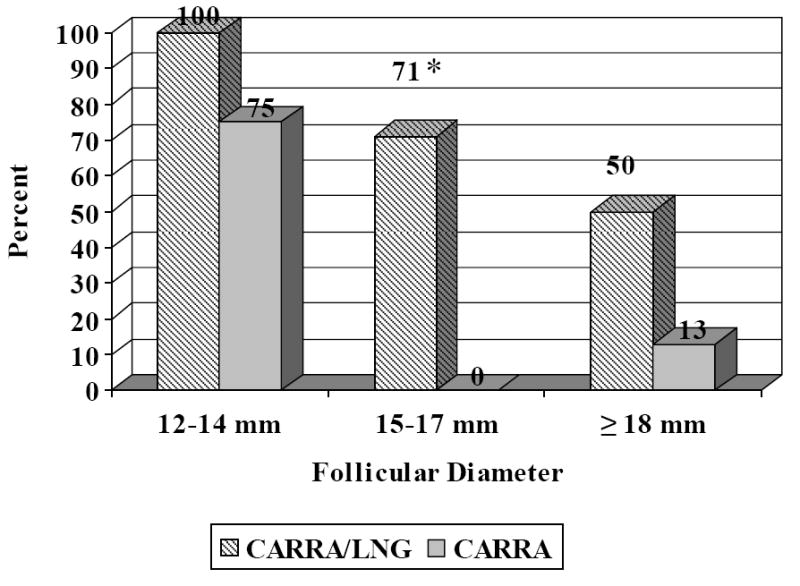
Percentage of cycles without follicular rupture in the 5-day period following treatment of CARRA/LNG 0.75 mg/4 mL or CARRA alone, according to follicular diameter at time of administration.
* p=0.0253 versus CARRA; McNemar’s chi-square test.
The total incidence of ovulatory dysfunction was 22% in the CARRA/LNG versus 9% in the CARRA cycles (Table 2). When lack of follicular rupture and ovulatory dysfunction in the 5-day period are added together, the overall proportion of cycles with either one of these conditions was 96 and 39% in the CARRA/LNG and CARRA cycles, respectively (p= 0.0008).
Ovulatory dysfunction was characterized by blunted or absent LH peak or LH elevation subsequent to follicular rupture (Fig. 2). While mean LH levels were slightly over 50 IU/mL on the day preceding follicular rupture in the ovulatory cycles of the CARRA gel group, this value was close to 10 IU/mL on the day preceding follicular rupture among the 5 subjects with ovulatory dysfunction in the CARRA/LNG cycles. Mean highest luteal P levels in this group was not different from those observed in the CARRA ovulatory group (51.6 ± 13.4 vs. 51.9 ± 19.1 nmol/L, respectively).
Fig. 2.
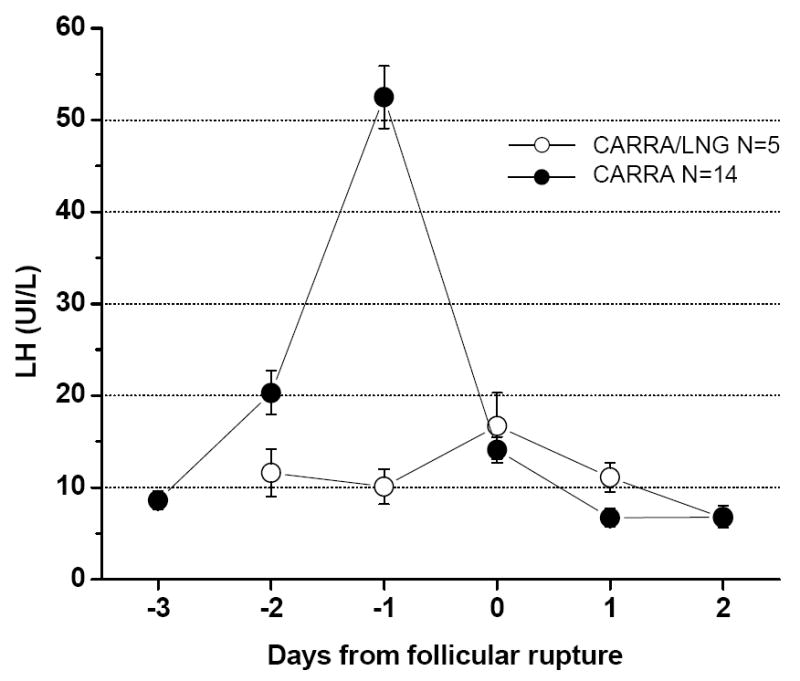
Mean LH levels in CARRA/LNG cycles with ovulatory dysfunction as compared to ovulatory CARRA cycles.
CARRA/LNG and CARRA cycles were significantly different regarding the outcome of follicles that did not rupture within the 5-day periods. In all 7 cycles in the CARRA group, rupture of the dominant follicle was observed within the first or second visit following the 5-day follow-up period, and all but one of these cycles corresponded to the 12-14 mm group. These follicles ruptured with a mean follicular diameter of 20.3 ± 2.7 mm (range 15.5-23.9). On the other hand, rupture was observed in only 6 of 17 cycles in the CARRA/LNG group [mean follicular diameter: 25.4 ± 6.5 mm (range 20.0-38.0)]. In the remaining 11 cycles, no subsequent ovulation occurred, instead the follicle underwent atresia (n=1) or developed into a persistent follicle (n=5) or a luteinized unruptured follicle (n=5).
3.2. Bleeding patterns
Mean cycle length was 28.3 ± 3.1 in the CARRA cycles and 30.3 ± 9.9 in the CARRA/LNG group. In the CARRA group, 91% of the cycles were of normal duration (24-32 days), compared to 70% in CARRA/LNG group. In this latter group, 13% had short cycles (range 11-23 days) while 17% had long cycles (range 36-56 days).
3.3. Adverse events
The most frequently reported adverse events among the CARRA/LNG and CARRA group, respectively, were headache (26 and 35%) and lower abdominal pain (13 and 17%), both of these unlikely to be related to product use.
3.4. LNG levels
In the CARRA LNG cycles, mean LNG levels were 4.1 ± 2.4 nmol/L at 24 h post gel application and dropped by half to 2.1 ± 1.2 nmol/L at 48 h, and to 1.1 ± 0.6 nmol/L at 72 h (Fig. 3). At 4 and 5 days after gel administration, 22/23 and 18/23 of the women, respectively, still had detectable LNG serum levels (> 0.15 nmol/L). LNG results were undetectable in all samples taken in the CARRA gel cycle.
Fig. 3.
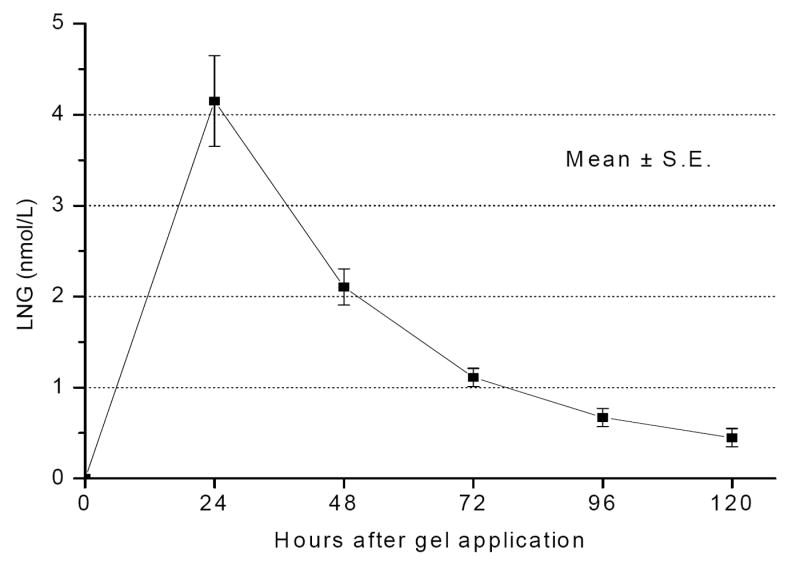
Mean LNG levels following vaginal administration of CARRA/LNG gel (0.75 mg/4 mL).
4. Discussion
The results of this study confirm that the vaginal administration of LNG in CARRA gel is effective for interfering with the ovulatory process when administered in the late follicular phase. Only 1 of 23 cycles had normal ovulation within the following 5 days after gel application. Moreover, this effect was maintained when the gel was administered with follicles ≥18 mm; only 1 of 8 cycles had normal ovulation.
It is interesting to note that the ability of CARRA/LNG 0.75 mg gel to adversely affect the ovulatory process was at least as effective, or better, than the oral administration of either 0.75 mg or 1.5 mg of LNG (as a single or split dose), previously reported by the same authors [12,13]. The percentage of cycles without follicle rupture was 50% after single oral administration of 0.75 mg and 44% after the standard 0.75 mg × 2 dose, as compared to 74% reported in this study. Similarly, the proportion without follicle rupture or with ovulatory dysfunction was 86% and 79% with the single or standard LNG oral dose as compared with 96% with CARRA/LNG [12]. As the subjects were different in the two studies, a direct comparison may not be valid, but it is at least a strong indication that the expected clinical effectiveness would not be lower, and possibly would be higher, than using the oral route.
The similar or stronger inhibitory effect on the ovulatory process observed with the vaginal administration of CARRA/LNG as compared to the oral intake of the same dose does not correspond with the LNG plasma concentrations observed following administration of these two products. Mean serum LNG levels on days 1-5 after treatment with the single oral 0.75 mg dose in the Croxatto et al. study [12] were between 30 and 60% higher than those observed in the present study after administration of the same dose via CARRA/LNG gel. Furthermore, pharmacokinetic studies indicate that the high peak that occurs 1-2 h after oral administration is missing after the administration of the vaginal gel [10, 14-16]. A lower Cmax and a later Tmax have similarly been reported with the vaginal route as compared with oral route of administration of LNG emergency contraceptive pills [17-19].
It may be that the oral dose currently used is unnecessarily high and the same effect can be obtained with much lower serum concentrations of LNG. Alternatively, we speculate that a higher local concentration of LNG in the uterus and the ovaries can be achieved after vaginal than after oral administration. The first uterine pass effect has been postulated, based on the much higher concentration of P in uterine tissue than in peripheral blood after vaginal administration of the steroid [20]. On the contrary, a recent publication comparing uterine concentrations of ethinyl estradiol and etonogestrel after use of a contraceptive vaginal ring and an oral contraceptive showed similar concentrations in myometrium and cervical tissues, but lower steroid concentrations in the endometrium in the vaginal ring group [21]. However, another study comparing serum and endometrial LNG levels following oral or vaginal administration of 1.5 mg LNG, found that in spite of much higher LNG serum levels achieved by the oral route, there was a trend for higher LNG endometrial levels in the women who received the vaginal dose, and the ratio between serum and vaginal LNG levels was lower in the vaginal group [19]. This first uterine pass effect can be explained by the extensive vascular connections between the vagina and the uterus [22]. The vascular connections between the uterus and the ovary are equally, if not more, extensive and it would be biologically plausible that the ovary is exposed to higher concentrations of a drug placed in the vagina, as compared with peripheral tissues, having a direct effect on the follicular milieu.
Independent of the mechanism that explains the effect of the vaginal administration of CARRA/LNG, the data presented here indicate little doubt that this product is a promising method for use as an emergency contraceptive method “on-demand” and has the potential for providing dual protection when used before coitus. Further studies are required to test the potential of pregnancy prevention of this combination gel as compared with approved methods and to evaluate the duration of the contraceptive efficacy if applied either immediately or several hours before intercourse. The alterations observed in the cycles, one third of which were shorter than 24 or longer than 32 days, are in agreement with the results found with the use of oral LNG emergency contraception [23]. It is an indication that this is not a method that can be used several times a month, before each sexual encounter but reserved for occasional use “on-demand”, as repeated use may induce cycle disruption. Frequent use of 0.75 mg LNG administered orally immediately after coitus, has been associated with more than 70% of women reporting menstrual irregularities. Furthermore, it is less effective than use of regular hormonal contraceptives [24].
Although the protection against STI provided by Carraguard is still not proven, if it were as expected, the CARRA/LNG gel could contribute to solve the dilemma frequently raised, suggesting that emergency contraception may discourage the use of condom and favor an increase in STI [25,26]. A method for emergency contraception prior to intercourse, such as CARRA/LNG, would provide protection against STI independent of the use of condoms and, hence, would eliminate that concern. Moreover, while the willingness of the male partner is required for use of the male condom, the use of CARRA/LNG gel would be controlled by women themselves.
The future of this potential woman-controlled dual protection agent depends on the confirmation of the microbicide effectiveness of Carraguard. If such effectiveness were demonstrated, CARRA/LNG would have great promise to become a powerful tool for the protection of women’s sexual and reproductive health. Alternatively, another effective microbicide with similar vaginal retention properties may be combined with the same dose of LNG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antiviral Res. 1988;9:335–43. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearce-Pratt R, Phillips DM. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of HIV-1. Biol Reprod. 1996;54:173–82. doi: 10.1095/biolreprod54.1.173. [DOI] [PubMed] [Google Scholar]
- 3.Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diag Lab Immunol. 1997;4:465–8. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire R, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–65. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Spencer SE, Valentin-Bon IE, Whaley K, Jerse AE. Inhibition of Neisseria gonorrhoeae genital tract infection by leading-candidate topical microbicides in a mouse model. J Infect Dis. 2004;189:410–9. doi: 10.1086/381125. [DOI] [PubMed] [Google Scholar]
- 7.Elias CJ, Coggins C, Alvarez F, et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception. 1997;56:387–9. doi: 10.1016/s0010-7824(97)00176-5. [DOI] [PubMed] [Google Scholar]
- 8.Coggins C, Blanchard K, Alvarez F, et al. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Inf. 2000;76:480–3. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilmarx PH, van de Wilgert JH, Chaikummao S, et al. Safety and acceptability of the candidate microbicide Carraguard in Thai women: findings from a Phase II clinical trial. J Acquir Immune Defic Syndr. 2006;43:327–34. doi: 10.1097/01.qai.0000243056.59860.c1. [DOI] [PubMed] [Google Scholar]
- 10.Sitruk-Ware R, Brache V, Maguire R, et al. Pharmacokinetic study to compare the absorption and tolerability of two doses of levonorgestrel following single vaginal administration of levonorgestrel in Carraguard® gel: a new formulation for “dual protection” contraception. Contraception. doi: 10.1016/j.contraception.2007.02.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy and sex of the baby. N Engl J Med. 1995;333:1517–21. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- 12.Croxatto HB, Brache V, Pavez M, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75 mg dose given on the days preceding ovulation. Contraception. 2004;70:442–50. doi: 10.1016/j.contraception.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Massai R, Forcelledo ML, Brache V, et al. Does meloxicam increase the incidence of anovulation induced by single administration of levonorgestrel in emergency contraception? A pilot study. Hum Reprod. 2007;22:434–9. doi: 10.1093/humrep/del369. [DOI] [PubMed] [Google Scholar]
- 14.Johansson E, Brache V, Alvarez F, et al. Pharmacokinetic study of different dosing regimens of levonorgestrel for emergency contraception in healthy women. Hum Reprod. 2002;17:1472–6. doi: 10.1093/humrep/17.6.1472. [DOI] [PubMed] [Google Scholar]
- 15.Tremblay D, Gainer E, Ulmann A. The pharmacokinetics of 750 μg levonorgestrel after administration of one single dose or two doses at 12- or 24-h interval. Contraception. 2001;64:327–31. doi: 10.1016/s0010-7824(01)00276-1. [DOI] [PubMed] [Google Scholar]
- 16.Kook K, Gabelnick H, Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception. 2002;66:73–6. doi: 10.1016/s0010-7824(02)00321-9. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez F, Faundes A, Johansson E, Coutinho E. Blood levels of levonorgestrel in women following vaginal placement of contraceptive pills. Fertil Steril. 1983;40:120–3. doi: 10.1016/s0015-0282(16)47189-x. [DOI] [PubMed] [Google Scholar]
- 18.Kives S, Hahn P, White E, Stanczyk FZ, Reid R. Bioavailability of the Yuzpe and levonorgestrel regimens of emergency contraception: vaginal vs. oral administration. Contraception. 2005;71:197–201. doi: 10.1016/j.contraception.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Devoto L, Fuentes A, Palomino A, et al. Pharmacokinetics and endometrial tissue levels of levonorgestrel alter administration of a single 1.5-mg dose by the oral and vaginal route. Fertil Steril. 2005;84:46–51. doi: 10.1016/j.fertnstert.2005.01.106. [DOI] [PubMed] [Google Scholar]
- 20.De Ziegler D, Bulletti C, De Monstier B, Jaaskelainen AS. The first uterine pass effect. Ann NY Acad Sci. 1997;828:291–9. doi: 10.1111/j.1749-6632.1997.tb48550.x. [DOI] [PubMed] [Google Scholar]
- 21.Roumen FJME, Dieben TOM. Comparison of uterine concentrations of ethinyl estradiol and etonogestrel after use of a contraceptive vaginal ring and an oral contraceptive. Fertil Steril. 2006;85:57–62. doi: 10.1016/j.fertnstert.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 22.Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82:1–12. doi: 10.1016/j.fertnstert.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–10. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- 24.WHO Task Force on Postovulatory Methods of Fertility Regulation. Efficacy and side effects of immediate postcoital levonorgestrel used repeatedly for contraception. Contraception. 2000;61:303–8. [PubMed] [Google Scholar]
- 25.Dupont S, Webber J, Dass K, Thornton S. Emergency contraceptive pill (ECP) and sexual risk behaviour. Int J STD AIDS. 2002;13:482–5. doi: 10.1258/09564620260079644. [DOI] [PubMed] [Google Scholar]
- 26.Fairhurst K, Ziebland S, Wyke S, Seaman P, Glasier A. Emergency contraception: why can’t you give it away? Qualitative findings from an evaluation of advanced provision of emergency contraception. Contraception. 2004;70:25–9. doi: 10.1016/j.contraception.2004.02.012. [DOI] [PubMed] [Google Scholar]


