Abstract
Objectives
Natural microbial defence systems, such as bacteriocins, may be a novel means to prevent catheter-associated urinary tract infection. We investigated in vitro whether a colicin-expressing strain of Escherichia coli could prevent urinary catheter colonization by a colicin-susceptible, uropathgenic strain of E. coli.
Methods
Segments of urinary catheter were inoculated with colicin-producing E. coli K-12 and then exposed to either colicin-susceptible E. coli (a uropathogenic clinical isolate) or colicin-resistant E. coli (derived from the susceptible clinical isolate). Catheters were then incubated overnight, rinsed and sonicated.
Results
The presence of colicin-producing E. coli K-12 on the catheter surface completely prevented catheter colonization by colicin-susceptible E. coli but not by resistant E. coli. The colicin-susceptible strain but not the colicin-resistant strain also disappeared from broth cultures in the presence of colicin-producing E. coli K-12.
Conclusions
The observed inhibition of catheter colonization by the uropathogenic clinical isolate of E. coli can be attributed to the presence of a colicin-producing strain of E. coli on the catheter surface. Bacteriocin production by a non-pathogenic organism may have clinical applicability as a means to prevent catheter-associated urinary tract infection.
Keywords: bacteriocins, UTIs, uropathogens
Introduction
Individuals who require indwelling urinary catheters for bladder drainage become chronically infected with urinary pathogens and suffer from recurrent urinary tract infections.1 Chronic indwelling urinary catheters become encrusted with biofilms of uropathogens, which continually seed the urine, and bacteria dwelling in biofilms are notoriously resistant to standard antimicrobial agents.2 Thus, antibiotics are not effective at preventing urinary tract infections in the chronically catheterized population, and frequent use of antibiotics in such persons leads to the emergence of resistant pathogens.3
The lack of effective strategies to prevent catheter-associated urinary tract infection has led to interest in novel approaches. Bacteriocins are highly specific, natural antibiotics produced by bacteria and are toxic only to bacteria closely related to the producing strain.4 Bacteriocins produced by Escherichia coli are termed colicins and are encoded in stable plasmids. Colicin proteins have three domains: a translocation domain (which moves the protein into the target cell), a receptor binding domain (which binds specific receptors on the target cell) and a killing domain (methods include pore-formation and nuclease activity). When initially synthesized by the producing cell, the killing domain is bound to an immunity protein that acts as a safety cap until removed. Killing specificities are determined by the receptor-binding domain.5 Since E. coli is one of the most common urinary tract pathogens, we investigated in vitro whether we could use a colicin-expressing strain of E. coli to prevent urinary catheter colonization by a colicin-susceptible strain of E. coli. We chose to work with colicin E2, in which the killing protein is a DNA endonuclease.6
Materials and methods
Bacterial strains
Four bacterial strains were used in these experiments: E. coli K-12 producing colicin E2 (K-12 Col+), E. coli K-12 that does not make colicins (K-12 Col−), a pathogenic clinical isolate of E. coli susceptible to colicin E2 (ColS) and a spontaneous mutant of ColS that was resistant to colicin E2 (ColR).7 The K-12 strains (from R. A. Hull’s collection) were lactose-negative, while the ColS and ColR strains were lactose-positive.
Colicin production by the K-12 Col+ strain was verified by stab plate experiments.7 Briefly, an agar plate was stabbed with a colony of E. coli K-12 Col+, incubated overnight at 37°C, exposed to chloroform to kill the K-12 Col+ bacteria, overlain with 108 cfu/mL of the test strain in soft agar (either ColS or ColR), then incubated again overnight.
Experimental protocol
Step 1. Trypticase soy broth (TSB; Becton Dickinson, Sparks, MD, USA) that contained two pieces of urinary catheter (Bardex, Lubricath Covington, GA, USA) was inoculated with 105 cfu/mL K-12 Col+ and then incubated overnight under static conditions at 37°C.
Step 2. One catheter piece was transferred to a tube containing ColS 105 cfu/mL in TSB for 30 min. The other catheter piece was transferred to a tube containing ColR 105 cfu/mL in TSB for 30 min.
Step 3. Both catheter pieces were transferred to separate 50 mL tubes of sterile TSB, and a broth sample from each tube was diluted and spread onto MacConkey agar (BBL, Cockeysville, MD, USA), which permitted ready discrimination of strains by colour. Tubes were incubated overnight under static conditions at 37°C.
Step 4. Catheters were removed from the tubes, rinsed in three separate baths of 50 mL of phosphate-buffered saline (PBS) and then flushed with PBS. Three 1 cm segments were cut from each catheter. Each 1 cm segment was placed in 1 mL o f PBS with 0.01% SDS and sonicated at 55 000 Hz for 10 min in a water bath sonicator (Buehler Scientific, Evanston, IL, USA) at room temperature.8 The sonication fluid was diluted and spread onto MacConkey agar. Broth from each tube in Step 4 was also diluted and spread onto MacConkey agar in order to evaluate the growth of the strains in mixed cultures. The experiment was repeated four times as above.
In two additional experiments, six catheters were used in Step 1. Two were exposed to K-12 Col+ (as before), two were exposed to K-12 Col−, and two were kept in sterile broth in Step 1. The purpose of incorporating the K-12 Col−-coated catheters and the uncoated catheters was to differentiate the effect of a pre-existing biofilm of E. coli K-12 from the effect of colicins produced by the E. coli K-12 on subsequent catheter colonization. Steps 2–4 were the same as in the preceding experiments. In all experiments, the median value of the three 1 c m catheter segments was used as the cfu/cm for that catheter.
Results
Colicin production
A clear zone >10 mm in diameter appeared in the lawn of ColS around the stab of K-12 Col+. No zone appeared in ColS around K-12 Col−, and ColR did not form zones around either K-12 Col+ or K-12 Col−. These findings suggest that ColS was killed or inhibited by cell-free colicin which had diffused into the agar.
Catheter results
In all six trials, the presence of colicin-producing E. coli on the catheter surface completely blocked catheter colonization by the colicin-susceptible E. coli; no susceptible E. coli was recovered from any catheter pre-coated with K-12 Col+ in six experiments (Figure 1). Colicin-resistant E. coli was able to colonize urinary catheters pre-coated with K-12 Col+; the median of ColR recovered from such catheters was 1900 cfu/cm (range 530–11 000 cfu/cm). This difference between ColS and ColR recovered from catheters pre-coated with colicin-producing E. coli K-12 was statistically significant (P = 0.009, Wilcoxon Rank Sum test, SAS® software version 8e). When the pre-coating strain did not produce colicin, or when no pre-coating strain was applied, catheter colonization by the colicin-susceptible and -resistant strains was similar (Figure 1). These results indicate that the inhibition of colicin-susceptible E. coli in the presence of K-12 Col+ was caused by the production of colicin.
Figure 1.
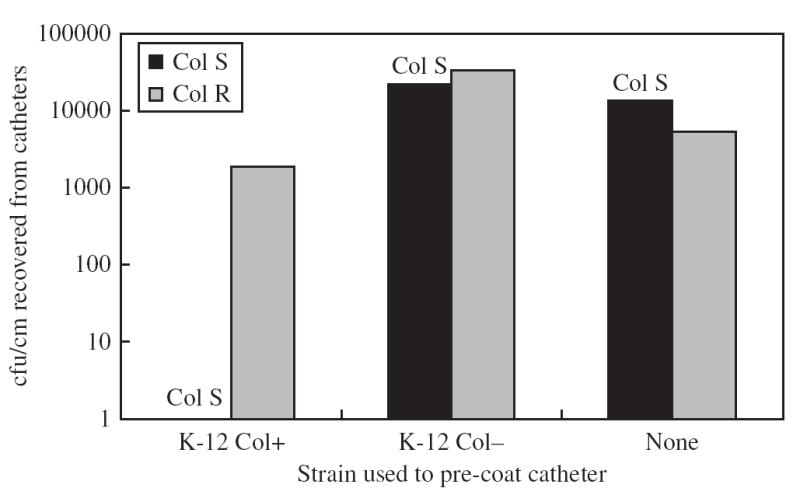
Recovery of challenge E. coli from treated catheters. The paired bars represent the medians of six, two and two experiments, respectively. No colicin-susceptible organisms were recovered from catheters pre-coated with the colicin producing strain in any of the six trials. The difference in the cfu/cm of ColS and ColR recovered from catheters coated with colicin-producing E. coli K-12 was statistically significant (P = 0.009, Wilcoxon Rank Sum test).
Mixed culture growth
In Step 3, catheters coated with K-12 Col+ that had been exposed to either ColS or ColR were transferred to sterile broth. Immediately after transfer, 205 cfu/mL of ColS and 440 cfu/mL of ColR (median values) were present in the broth (P = 0.10 for ColS compared with ColR, Wilcoxon Rank Sum test). After overnight incubation in the presence of K-12 Col+, 0 cfu/mL of ColS and 6 × 107 cfu/mL of ColR (median values) were recovered from the broth (P = 0.009, Wilcoxon Rank Sum test). These results represent six repetitions of the experiment.
Conclusions
Coating a urinary catheter with a colicin-producing strain of E. coli K-12 completely prevented catheter colonization by a susceptible clinical isolate but not by a resistant isolate. Disappearance of ColS but not ColR was also noted in broth in the presence of K-12 Col+. Our study had two limitations. First, each species of bacteria produces hundreds of unique microbicins,4 and our study investigated only one microbicin, colicin E2. We did preliminary catheter trials with colicin IB against E. coli ColS and ColR, and we also tested pyocin S1 against a clinical isolate of Pseudomonas aeruginosa.5 In neither case did we observe a significant effect of the microbicin upon catheter colonization by the ‘susceptible’ strain (B. W. Trautner and R. A. Hull, unpublished data). Of note, the zones in the stab plates for colicin IB and pyocin S1 were both hazy rather than clear, in contrast to the clear zones around colicin E2. Perhaps we can use the stab plate results to guide our choice of microbicins for future catheter studies.
A second limitation is that we used broth rather than urine as the growth medium. We chose broth for these experiments because the composition of human urine is more variable than broth, and artificial urine does not fully replicate the composition of human urine.
Currently the only bacteriocin in practical use is nisin, a Gram-positive bacteriocin with a broad killing range. Nisin is widely used in the food industry as a preservative.4 However, under controlled research conditions, colicins effectively cleared E. coli from the urine of rats,9 and colicins inhibited the growth of two strains of E. coli that cause swine diarrhoea.10 Our goal is to create a non-pathogenic strain of E. coli that simultaneously expresses bacteriocins active against a variety of uropathogens. We are currently investigating the use of urinary catheters coated with non-pathogenic E. coli to colonize the bladders of persons with spinal cord injury who suffer from recurrent urinary tract infections. Modifying our non-pathogenic strain to produce a spectrum of bacteriocins would have potential clinical applicability.
Acknowledgments
This work was supported by the Department of Veterans Affairs Rehabilitation Research and Development Service Merit Review #B2125-RA, Paralyzed Veterans of America Grant 302, and USPHS Grant HD42014.
References
- 1.Warren JW, Tenney JH, Hoopes JM, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719–23. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 2.Denstedt JD, Wollin TA, Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493–500. doi: 10.1089/end.1998.12.493. [DOI] [PubMed] [Google Scholar]
- 3.Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22:316–21. doi: 10.1086/501908. [DOI] [PubMed] [Google Scholar]
- 4.Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol. 2002;56:117–37. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 5.Kageyama M, Kobayashi M, Sano Y, et al. Construction and characterization of pyocin-colicin chimeric proteins. J Bacteriol. 1996;178:103–110. doi: 10.1128/jb.178.1.103-110.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller K, Nomura M. Colicin E2 is a DNA endonuclease. Proc Natl Acad Sci. 1976;73:3989–93. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredericq P. Colicins. Ann Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- 8.Sherertz RJ, Raad II, Belani A, et al. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braude AI, Siemienski JS. The influence of bacteriocins on resistance to infection by gram-negative bacteria. II. Colicin action, transfer of colicinogeny, and transfer of antibiotic resistance in urinary infections. J Clin Invest. 1968;47:1763–73. doi: 10.1172/JCI105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stahl CH, Callaway TR, Lincoln LM, et al. Inhibitory activity of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob Agents Chemother. 2004;48:3119–21. doi: 10.1128/AAC.48.8.3119-3121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


