Abstract
Ceramides are sphingolipids that greatly stabilize ordered membrane domains (lipid rafts), and displace cholesterol from them. Ceramide-rich rafts have been implicated in diverse biological processes. Because ceramide qnalogues have been useful for probing the biological function of ceramide, and may have biomedical applications, it is important to characterize how ceramide structure affects membrane properties, including lipid raft stability and composition. In this report, fluorescence quenching assays were used to evaluate the effect of analogues of ceramide with different N-acyl chains or different sphingoid backbones on raft stability and sterol content. The effect of replacing 18 mol% of sphingomyelin (SM) with ceramide in vesicles composed of a 1:1 (mol:mol) mixture of SM and dioleoylphosphatidylcholine (DOPC) with or without 25 mol% sterol, was examined. In the absence of sterol, the thermal stability of the SM-rich ordered domains increased with ceramide N-acyl chain length in the order C2:0 ~ C6:0 ~ C8:0 <no ceramide <C12:0 <C16:0. In vesicles containing 25 mol% cholesterol (1:1:0.66 sphingolipid:DOPC:cholesterol), the dependence of raft stability on ceramide N-acyl chain length increased in the order C8:0 <C6:0 <C2:0 <C12:0 <no ceramide <C16:0. We also studied the stability of lipid rafts in the presence of N-lauroyl- and N-palmitoylsphingosine analogues containing altered structures in or near the polar portion of the sphingoid base. In almost all cases, the analogues stabilized rafts to about the same degree as a normal ceramide containing the same acyl chain. The only exception was N-palmitoyl-4D-ribophytosphingosine, which was very strongly raft-stabilizing. We conclude that variations in sphingoid base structure induce only insignificant changes in raft properties. N-lauroyl- and N-palmitoylsphingosine and their analogues displaced sterol from rafts to a significant degree. Both C12:0 and C16:0 analogues of ceramide may be good mimics of natural ceramide, and useful for cellular studies in which maintenance of the normal physical properties of ceramide are important.
Introduction
Ceramide forms the structural backbone of most sphingolipids. Free ceramide is present in cell membranes and occurs in high concentrations in the stratum corneum of the human skin. It can be generated in vivo by ceramide synthase, by enzymatic degradation of glucosylceramide and galactosylceramide, or by sphingomyelinase (SMase)-catalyzed hydrolysis of sphingomyelin. SMases have been reported to be activated in many pathways that regulate processes such as apoptosis [1–3], B and T cell activation [4, 5], and TNFα-mediated-signaling [6–8]. Ceramide is also generated by SMases upon infection by several bacterial and viral pathogens [9–12]. Under some conditions it has been reported that activation of SMase leads to the generation of ceramide-rich membrane domains (ceramide-rich lipid rafts) on the plasma membrane, which can exist in the form of large ceramide-rich platforms [10, 13]. The formation and functional role of ceramide-rich domains have been reviewed recently [14].
Lipid rafts are tightly packed, ordered membrane domains that are generally enriched in both lipids with saturated acyl chains (e.g., sphingolipids) and sterol [15, 16]. They are believed to segregate from, and co-exist in eukaryotic membranes with, disordered membrane domains that are rich in lipids with unsaturated acyl chains [15]. Natural ceramide has a long saturated N-acyl chain and small polar headgroup, making it a lipid with very tight packing properties, as illustrated by the fact that model membranes composed of ceramide have a high order-to-disorder transition temperature [17]. Thus, it is not surprising that ceramide has the ability to stabilize and promote raft formation in model membranes [18, 19]. In addition, we recently observed that both pure C18:0-ceramide and natural ceramide mixtures have the ability to displace sterols from rafts [19]. Displacement of sterol by ceramide has been confirmed in other studies using both model and cellular membranes [20, 21] and appears to be of relevance for the constitutive degradation of intralysosomal membranes [22]. Displacement of sterol could result in ceramide having marked effects on lipid raft composition and physical properties.
In this report, we studied how the raft-stabilizing properties of ceramide are influenced by structural features in the ceramide molecule. A tempo-based fluorescent quenching assay was used to measure the thermal stability of ordered domains in model membrane vesicles composed of a mixture of ordered and disordered domain forming lipids [23]. First, the influence of N-acyl chain length on ceramide-induced stabilization of ordered domains was measured in bilayers with or without cholesterol. Then, the raft-stabilizing abilities of a series of synthetic ceramide Analogues with various structural alterations in or near the polar headgroup were compared (Figure 1). In addition, the ability of ceramide analogues to displace cholesterol from ordered domains was measured. The raft-stabilizing and sterol-displacing properties of many ceramide analogues were very similar to those of natural ceramide. These observations have important implications for the design of biomedically useful analogues of ceramide.
Figure 1.
Structures of the ceramide analogues examined in this study. Scientific names of the Analogues are as follows: compound 1 N-[(1S,2R,3E)-2-Hydroxy-1-(hydroxymethyl)-2-methylheptadec-3enyl]dodecanamide, compound 2 N-[(1S,2R)-2-Hydroxy-1-(hydroxymethyl)2-methylheptadecyl]dodecanamide, compound 3 N-[(1S,2R,3Z)-2-Hydroxy-1(hydroxymethyl)-2-methylheptadec-3enyl]dodecanamide, compound 4 - N-[(1S,2R)-2-Hydroxy-1-(hydroxymethyl)-2-methylheptadec-3ynyl]dodecanamide, compound 5 (rac-(2’tridecylcyclopropyl)-(3R)-3-hydroxy-(2S)-2-(dodecanamido)propanol), compound 6 (rac-1(5’,5’-dimethyl-1’,3’-dioxan-2’-yl)-1-(dodecanoylamino)-(E)-pentadec-3-en-2-ol, compound 7 (2S,3R)-2-palmitoylamido-4-octadecyne-1,3-diol, compound 8 (2S,3R)-3-(3'-Dodecylphenyl)-2-palmitoylamidopropane-1,3-diol, compound 9 (2S,3R,5R)-2-palmitoylamidooctadeca-4,5-diene1,3-diol, compound 10 (2S,3R)-2-palmitoylamido-(7E)-octadecene-1,3-diol, compound 11 (2S,3S,4S)-N-palmitoylphytosphingosine.
Materials and Methods
Materials
Dipalmitoylphosphatidylcholine (DPPC); porcine brain sphingomyelin (SM); cholesterol; C2:0-, C6:0-, C8:0-, C12:0-, and C16:0-ceramides; dioleoylphosphatidylcholine (DOPC); and 1-palmitoyl-2-(12-doxyl)-stearoylphosphatidylcholine (12-SLPC) were purchased from Avanti Polar Lipids (Alabaster, AL). Figure 1 shows the structures of the ceramide analogues studied. Compounds 1 and 4 and compounds 7–11 were synthesized as described in references [24–27]. The 3-C-methylceramides 2 and 3 were prepared as described previously [27]. Compound 5 (rac-(2’-tridecylcyclopropyl)-(3R)-3-hydroxy-(2S)-2-(dodecanamido)propanol) was prepared by cyclopropanation of N-lauroyl-sphingosine according to a modification of the Simmons-Smith reaction [28, 29]; compound 6 (rac-1-(5’,5’-dimethyl1’,3’-dioxaN-2’-yl)-1-(dodecanoylamino)-(E)-pentadec-3-en-2-ol; was prepared by nitro aldol addition [30] of 2,2-dimethyl-5-(nitromethyl)-1,3-dioxane to tetradecanal, followed by catalytic hydrogenation and N-acylation (P. Sawatzki, Ph.D. Thesis, University of Bonn, 2003). Lipid purity was confirmed by TLC as described previously [31]. 2,2,6,6-Tetramethylpiperidine-1-oxyl (Tempo) and dehydroergosterol (DHE) were purchased from Sigma-Aldrich (St. Louis, MO). N-(22-(Diphenylhexatrienyl)docosyl)-N,N,N-trimethylammonium iodide (long-chain TMA-DPH, Lc-TMADPH) was a kind gift from G. Duportail and D. Heissler (Université Louis Pasteur, Strasbourg).
Vesicle preparation
Ethanol dilution small unilamellar vesicles (SUVs) were prepared as described previously [19]. Vesicles containing the desired lipid mixture and 0.1 mol% Lc-TMADPH were dispersed at 70°C in PBS (10 mM Na phosphate, 150 mM NaCl, pH 7.0) at a final lipid concentration of 50 µM, and then cooled to room temperature. Background samples lacking Lc-TMADPH were prepared similarly.
Tempo quenching assay to measure raft stability stability
To “F samples” containing SUVs of the desired lipid composition an aliquot of a 353 mM solution of Tempo dissolved in ethanol was added. The final Tempo concentration was 2 mM. The same volume of ethanol lacking Tempo was added to prepare “Fo samples.” Tempo quenching of Lc-TMADPH fluorescence (excitation wavelength 357 nm; emission wavelength 429 nm) was measured on a Fluorolog 3 spectrofluorimeter (Jobin-Yvon, Edison, NJ) as a function of temperature as described previously [23].The ratio of fluorescence intensity in the F sample to that in the Fo sample (F/Fo) was calculated after the fluorescence in background samples were subtracted [32].
Displacement of sterol from ceramide-rich rafts rafts
DHE quenching by 12-SLPC was used to monitor displacement of sterol from rafts as described previously [19]. Briefly, ethanol dilution vesicles composed of 3:3:1:1 DPPC:DOPC:cholesterol:DHE (mol:mol) (Fo samples) or 3:3:1:1 DPPC:12-SLPC:cholesterol:DHE (F samples) were prepared as described above. 12SLPC is a fluorescence quenching lipid that behaves similarly to DOPC [32, 33]. F/Fo was calculated after subtracting the light scattering in background samples containing cholesterol in place of DHE. DHE fluorescence was measured using an excitation wavelength of 324 nm and emission wavelength of 376 nm.
Results
Raft-stabilizing ability of ceramides with varying N-acyl chain lengths
A fluorescence quenching assay employing the nitroxide-containing Tempo molecule was used to assess raft formation [23]. Model membrane vesicles containing a fluorescent molecule and, a lipid mixture that tends to form co-existing ordered and disordered domains were prepared. The fluorescent probe Lc-TMADPH, which partitions favorably into ordered phases, was used [18, 19, 31]. Tempo binds to disordered domains much more strongly than to ordered domains, so that the quenching of Lc-TMADPH is weak when ordered domains are present, but strong when the membrane is fully in a disordered state [31, 34]. To study ordered domain/raft stability, we measured quenching of Lc-TMADPH fluorescence by Tempo as a function of temperature. Ordered domains melt at a high temperature, and on melting mix with the lipids in the disordered domains. An ordered domain-melting temperature (Tm) can be derived from this experiment [31, 32]. The higher the Tm, the more stable the rafts.
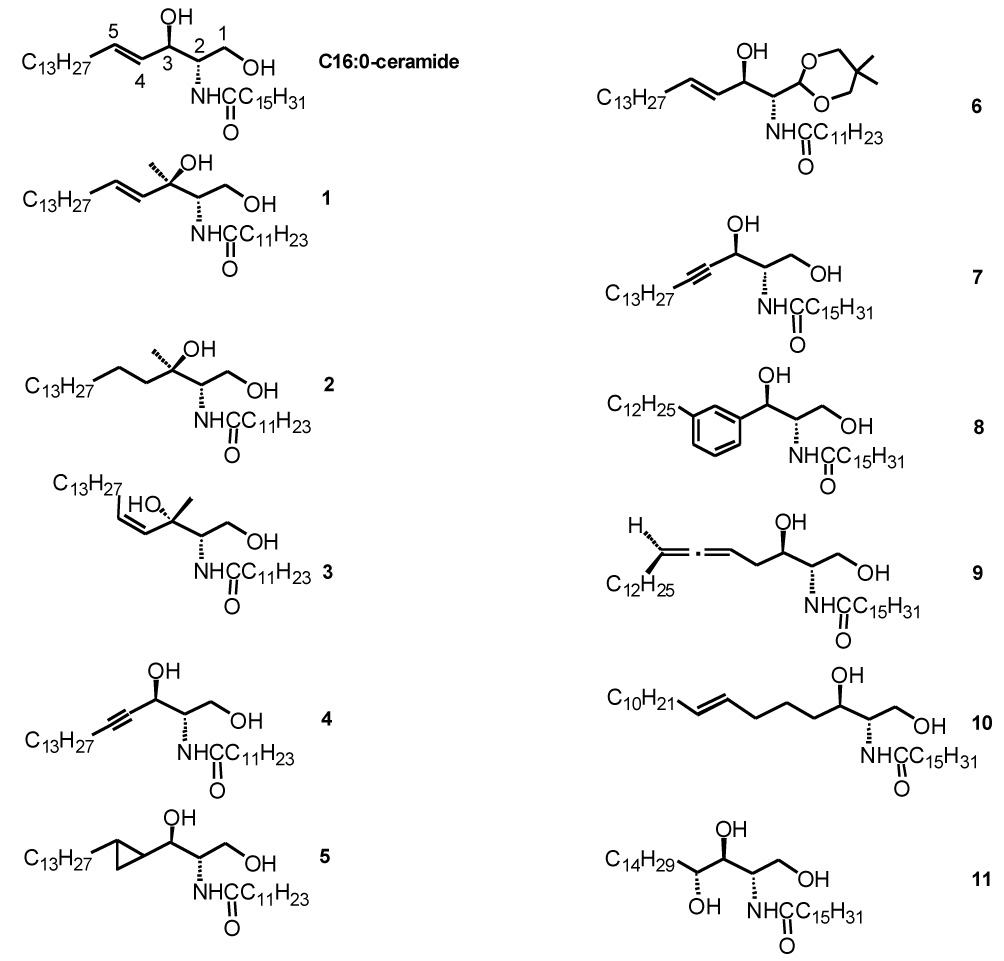
We first determined the effect of ceramides on the stability of ordered domains in mixtures containing equimolar sphingolipid and DOPC (32:18:50 SM:ceramide:DOPC (mol:mol)) (Figure 2A). Compared with a sample lacking ceramide (50:50 SM:DOPC), short-chain ceramides (those with a C2:0, C6:0, or C8:0 N-acyl chain) destabilized ordered domains (Figure 2A and Table 1), indicating that the short-chain ceramides are less effective than SM in stabilization of ordered domains. The stability of the ordered domains was enhanced by C12:0-ceramide, and to an even greater degree by C16:0-ceramide. Ordered domain stability in the presence of C16:0-ceramide was similar to that measured previously for C18:0-ceramide in a slightly different lipid mixture [19]. This shows that longer chain ceramides stabilize ordered domain formation.
Figure 2.
Effect of ceramides on raft stability as assessed from the effect of temperature on quenching of Lc-TMADPH by Tempo. Vesicles were composed of A) 32:18:50 SM:ceramide:DOPC (mol%) and B) 19.5:18:37.5:25 SM:ceramide:DOPC:cholesterol. Key: C2:0 cer (open circle), C6:0 cer (open triangle), C8:0 cer (plus sign), C12:0 (filled black circle), C16:0 cer (filled triangle), and no ceramide (filled gray circle). “None” refers to 50:50 total sphingolipid:DOPC. Results shown are average values from duplicates. Quenching data were fit to sigmoidal curves using the program Slidewrite for this and the following figures (see Table 1 for details). Samples in this and the following figures contained 50 nmol of total lipid in 1 ml of PBS). Cn:0 = Cn:0 ceramide, where n equals N-acyl chain length.
Table 1.
Tm (melting temperature) of ordered domains in vesicles containing ceramides with various N-acyl chain lengths. Data from the experiments shown in Figure 2 were fit to a sigmoidal curve (Slidewrite program, Advanced Graphic Software, Encinitas, CA) and then Tm was calculated from the mid-point of the curve. The values shown are the average values and the range from duplicate samples. For the lipid composition of the vesicles, see the caption to Figure 2. In the cases in which a Tm range is shown melting curves were too incomplete to give a sigmoidal fit with a physically meaningful limiting quenching value (i.e. F/Fo<1) at low temperature. In this case the Tm range shown (in brackets) is for the maximum and minimum possible low temperature limiting F/Fo values. The maximum limiting F/Fo value is 1, and the minimum F/Fo value is the experimental value at the lowest temperature measured. The midpoint of this range may be considered a useful approximation to the actual Tm.
| Ceramide | Tm (°C) | |
|---|---|---|
| -chol | +25 mol% chol | |
| None | 25.5±0.2 | 34.0±0.4a |
| C2:0 | 19.1±2.8 | 31.7±0.3 |
| C6:0 | [9.4–21.9] | [25.6–31.4] |
| C8:0 | 19.3±3.4 | [23.1–31.1] |
| C12:0 | 37.6±0.5 | 33.4±0.7 |
| C16:0 | 46.9±0.0 | 47.8±0.5 |
Data from reference [23].
In the above samples, ceramide replaced some of the SM, so that the SM content was lower than in the ceramide-containing samples. We repeated these experiments using samples in which ceramide replaced some of the DOPC (50:18:32 SM:ceramide:DOPC), so that the mol% SM in ceramide-containing samples was the same as that in ceramide-lacking samples. In these samples, the Tm values for vesicles containing C6:0- and C8:0-ceramide were still found to be slightly lower than the Tm value in the absence of ceramide, showing that these short-chain ceramides have an effect on ordered domain stability that is similar to that as DOPC (data not shown).
Canonical model membranes containing ordered domains mimicking rafts contain cholesterol (chol); therefore, we determined if these ceramides affected raft stability in vesicles containing chol. Samples containing ceramide were composed of 19.5:18:37.5:25 (mol:mol) SM:ceramide:DOPC:chol, and those without ceramide were composed of 37.5:37.5:25 SM:DOPC:chol. Note that ceramide forms a larger fraction of the total sphingolipid (SM+ceramide) than in the samples lacking sterol.
In most cases, rafts (we will use the term “raft” for ordered domains in samples containing chol) were more stable in the presence of chol than ordered domains in the absence of chol (Figure 2B and Table 1). This is in agreement with previous studies showing that chol stabilizes ordered domain formation [16, 18, 32, 35–37]. However, rafts were not stabilized by chol in samples containing C12:0- and C16:0-ceramide (the Tm is about 4°C lower than in the absence of chol). This can be explained by the observations that: (a) these ceramides prevent strong association of chol with rafts (see below), and (b) the chol that does associate with rafts partially displaces ceramide from the rafts [19]. Thus, this result suggests that the tendency the chol that can associate with ceramide-rich rafts to increase raft stability is insufficient to compensate for the loss of raft stability because of partial displacement of ceramide from rafts by chol.
The order of the effect of ceramide N-acyl chain length on raft stability in the presence of chol (C16:0>C12:0>C2:0>C6:0>C8:0) is similar to that found in the absence of chol (Table 1). Again, the C2:0-, C6:0-, and C8:0-ceramides decreased ordered domain stability relative to that in samples lacking ceramide, whereas C16:0-ceramide increased ordered domain stability relative to that in the absence of ceramide (Figure 2B and Table 1). However, unlike the situation in the absence of chol, C12:0-ceramide did not stabilize raft formation relative to that in the absence of ceramide (Tm 38°C in the absence of C12:0-ceramide and 34°C in its presence). This implies that the increase in raft stability from raft-association by C12:0-ceramide is insufficient to compensate for the loss in raft stability from displacement of chol by C12:0-ceramide (see below).
Raft-stabilizing abilities of ceramide analogues
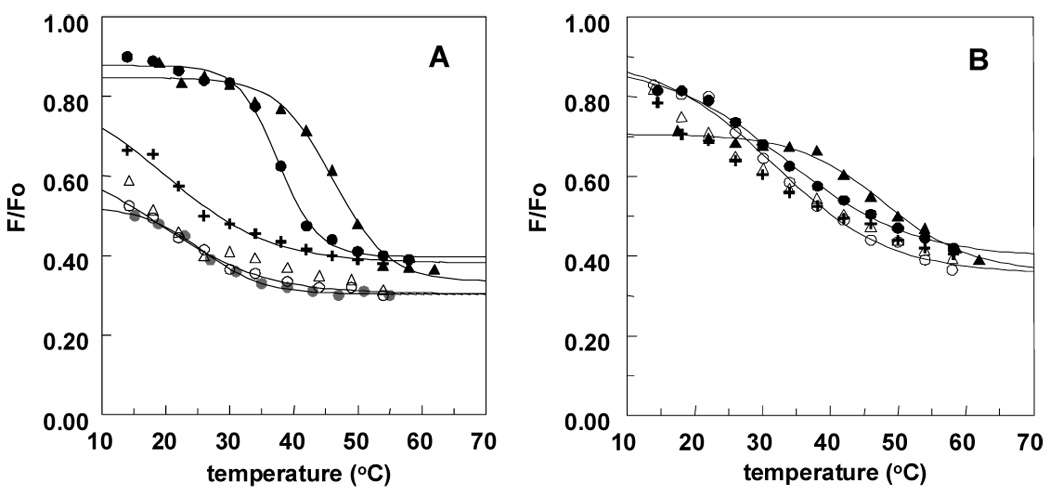
The effect of a series of ceramide analogues on the stability of rafts was studied. We tested analogues of C12:0- and C16:0-ceramide because, as shown above, the presence of a long N-acyl chain is required to maintain stable ordered domains. The Tempo quenching assay was first used to analyze the raft-stabilizing abilities of several C12:0-ceramide analogues in vesicles composed of 19.5:18:37.5:25 (mol:mol) SM:ceramide:DOPC:chol (Figure 3 and Table 2). Vesicles containing an analogue with a C3-methyl group in the sphingoid base (compound 1) had a similar raft stability as C12:0-ceramide (compare Table 1 and 2). We also observed similar Tm values for the stereoisomer of compound 1, with an S configuration at C3 (data not shown). In contrast, relative to C12:0 ceramide, an analogue with a cyclopropyl group bridging C4 and C5 somewhat stabilized raft formation (compound 5), while an analogue with a combination of a C3-methyl and a 4,5 cis double bond destabilized raft formation to a significant degree (compound 3). Raft stability in the presence of analogs containing a replacement of the primary hydroxyl group with a relatively bulky dimethyl-1,3-dioxanyl group (compound 6), or containing a 4,5 single bond (compound 2) or 4,5 triple bond (compound 4) seemed to differ only slightly from that in the presence of C12:0 ceramide.
Figure 3.
Effect of analogues of C12:0-ceramide on raft stability as assessed from the effect of temperature on quenching of Lc-TMADPH by Tempo. Vesicles were composed of 19.5:18:37.5:25 SM:ceramide analogue:DOPC:chol. Key: Panel A: compound 1 (plus sign), compound 2 (open triangle), compound 3 (open circle) and C12:0 cer (filled circle); Panel B: compound 4 (open triangle), compound 5 (open circle) and compound 6 (plus sign) Results shown are the average values from duplicates. C12:0 = C12:0-ceramide
Table 2.
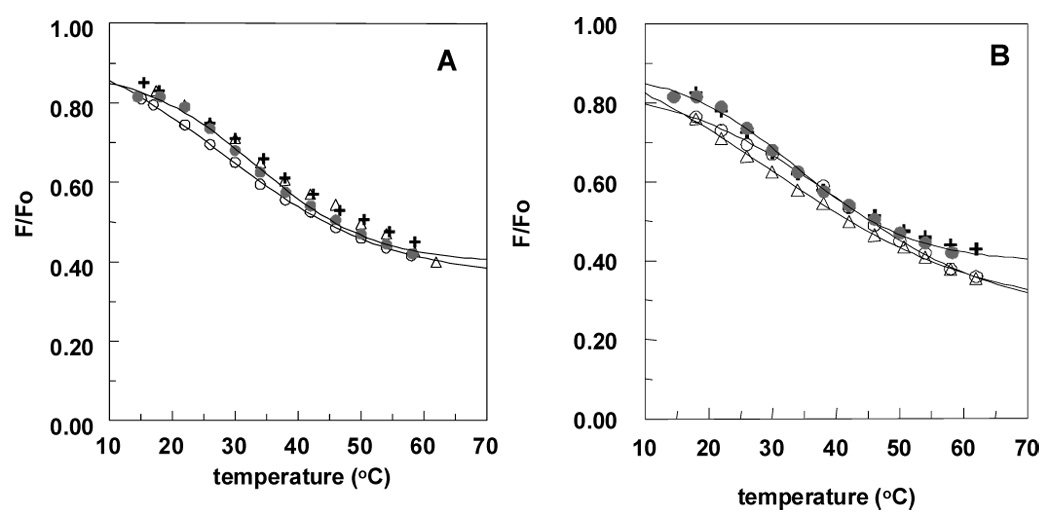
Tm (melting temperature) of ordered domains in vesicles containing ceramide analogues and 25mol% cholesterol. Lipid compositions as given in Figure 3 and Figure 4. Data analyzed as described in Table 1. In the cases in which a Tm range is shown for the C12:0 analogues, melting curves were too incomplete to give a sigmoidal fit with a physically meaningful limiting quenching value (i.e. F/Fo<1) at low temperature, and were analyzed as described in Table 1. In the cases in which a Tm range is shown for the C16:0 analogues, melting curves were too incomplete to give a sigmoidal fit with a physically meaningful limiting quenching value (i.e. F/Fo>0) at high temperature, and were analyzed as described in Table 1. In this case the Tm range shown (in brackets) is for the maximum and minimum possible low temperature limiting F/Fo values. The minimum limiting F/Fo value is 0, and the minimum F/Fo value is the experimental value at the highest temperature measured.
| Ceramide Analogue | Tm (°C) |
|---|---|
| C12:0 compounds | |
| 1 | 32.4±0.1 |
| 2 | [34.5–38.4] |
| 3 | 28.4±0.4 |
| 4 | [29.2–36.7] |
| 5 | 40.2±0.3 |
| 6 | [28.0–33.6] |
| C16:0 compounds | |
| 7 | 40.6±0.3 |
| 8 | 43.2±0.2 |
| 9 | [42.4–50.2] |
| 10 | 42.8±0.2 |
| 11 | [54.0–63.2] |
Raft stability was also assessed for a series of analogues of C16:0-ceramide (compounds 7–11). As shown in Figure 4 and Table 2, raft stability in the presence of compound 7 was somewhat less, and in the presence of compounds 8–10 was at most slightly less, than that in the presence of C16:0-ceramide (compare Table 2 and Table 1). The most striking result was a large increase in raft stability, relative to that in the presence of C16:0 ceramide, observed in vesicles containing phytoceramide (compound 11) (see Figure 4). Also notice that, as for the parental C16:0 and C12:0 ceramides, the C16:0 analogues all give higher Tm than the C12:0 analogues.
Figure 4.
Effect of analogues of C16:0-ceramide on raft stability as assessed from the effect of temperature on quenching of Lc-TMADPH by Tempo. Vesicles were composed of 19.5:18:37.5:25 SM:ceramide analogue:DOPC:chol. Key: compound 7 (plus sign), compound 8 (triangle), compound 9 (open circle), compound 10 (square), compound 11 (inverted triangle) and C16:0 cer (filled circle). Quenching curves for compounds 9 and 11 were assigned a limiting F/Fo value of 0.3 at high temperature. Results shown are the average values from duplicates. C16:0 = C16:0-ceramide
Displacement of sterol from ordered domains by various ceramide analogues
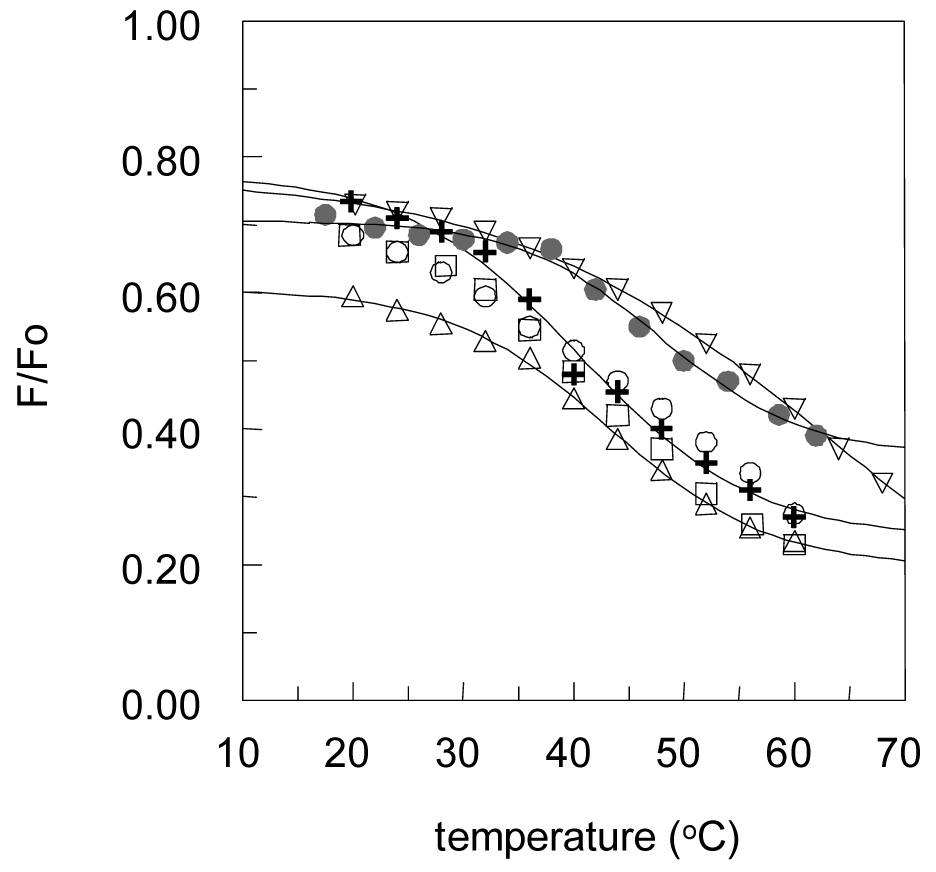
We previously found that ceramide is able to displace sterols from rafts [19] and sought to determine whether ceramide structure would affect displacement. For this study, we examined the displacement of the fluorescent sterol dehydroergosterol (DHE) from rafts as measured by a different fluorescent quenching assay. This assay detects the displacement of DHE from ordered domains by the fact that the displaced DHE moves into disordered domains enriched in the nitroxide-labeled quencher lipid 12SLPC [19]. Thus, ceramide-induced displacement results in an increase in quenching (decrease in F/Fo) in the presence of the ceramide. A decrease in F/Fo of 50% very roughly corresponds to almost complete displacement of sterol from the ordered domains [19]. As shown in Figure 5A and 5B, both C12:0- and C16:0-ceramide derivatives have the ability to strongly displace DHE from ordered domains. There was no significant difference in sterol displacement tendency with the different analogues that could be detected at the ceramide analogue concentration used.
Figure 5.
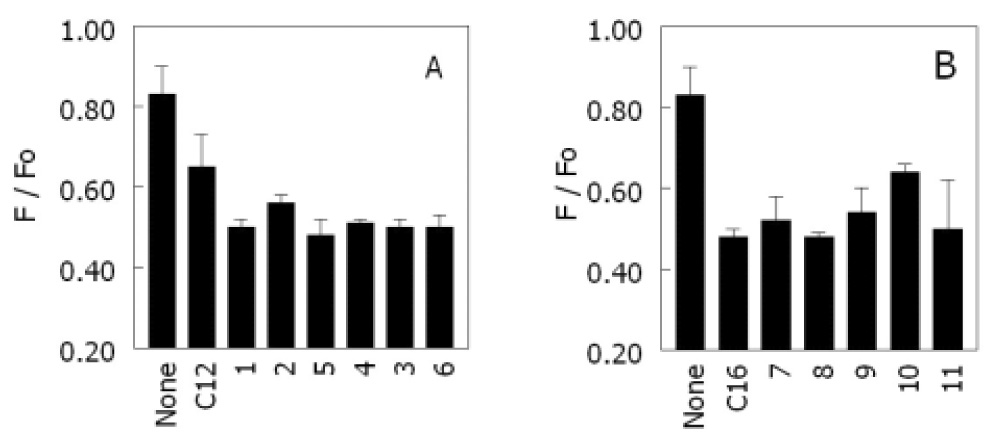
Displacement of dehydroergosterol (DHE) by various ceramide analogues: A) C12:0-ceramide and its Analogues. B) C16:0-ceramide and its analogues. Samples containing ceramide or ceramide Analogues were composed of 19.5:18:37.5:12.5:12.5 DPPC:ceramide:12-SLPC:DHE:chol (F samples) or the same mixture with DOPC in place of 12-SLPC (Fo samples). Samples without ceramide were composed of 37.5:37.5:12.5:12.5 DPPC:12-SLPC:DHE:chol (F samples) or the same mixture with DOPC in place of 12-SLPC (Fo samples). Average values and the range from duplicate samples are shown.
Discussion
Structural features of ceramide that influence raft stability
This study shows that raft stability is related to ceramide N-acyl chain length. A minimum of a C12:0 N-acyl chain length was required for significant raft stability. It is known that phospholipids with one long and one short acyl chain have low Tm values, indicative of the inability to form thermally stable ordered domains [38]. Because the long alkyl group of the sphingoid base of ceramide is equivalent to a long hydrocarbon chain, short-chain ceramides are analogous to lipids with one long chain. Thus, the inability of short-chain ceramides to stabilize ordered domains formation is not surprising. The conclusion that N-acyl chain structure is important for the ability of ceramides to form thermally stable ordered domains is further supported by our previous observation that N-oleoylsphingosine disrupts ceramide’s raftstabilizing ability [19, 39].
Our observations with the short-chain ceramides in cholesterol-containing vesicles are in close agreement with the very recent study of Nybond et al. [40], who measured raft stability by measuring the quenching of a sphingomyelin containing a fluorescent parinaroyl fatty acyl chain by a nitroxide acyl chain-labeled PC. One difference involves C2:0-ceramide. Both studies concluded that C2:0-ceramide is less raft-destabilizing than C6:0- and C8:0-ceramide; however, Nybond et al. found C2:0-ceramide to be raft-stabilizing, while we found it to be raft-destabilizing. The difference in the two studies could be related to differences in the acyl chain structure of the lipid used (POPC and C16:0-SM in Nybond et al. [40] vs. DOPC and brain SM in our experiments) and/or to differences in the lipid composition (10 mol% chol and a higher PC content in Nybond et al. [40] vs. 25 mol% chol and a lower PC content in our experiments).
It should also be noted that the absolute values of Tm could be affected by a number of factors. For example, vesicle size can affect Tm slightly [41], as can the partition coefficient of the fluorescent probe between fluid and ordered domains. In this study we used LcTMADPH, which partitions favorably into ordered domains [42], because unlike DPH which partitions more equally between ordered and disordered domains, LcTMADPH is not displaced from ceramide-rich ordered domains [19]. The use of LcTMADPH give Tm values a couple of degrees higher than does DPH, but this does not affect relative Tm values significantly [31].
Our studies also show that the effects of ceramide N-acyl chain length on ordered domain stability are qualitatively similar in the absence and presence of chol. This indicates that the effect of N-acyl chain length on tight lipid packing is crucial to the ability of ceramide to stabilize rafts, and that the ability of ceramide to stabilize rafts does not involve a specific ceramide-sterol interaction.
The behavior of C12:0- and C16-ceramide analogues reveals that most small modifications near the polar headgroup of the sphingoid base do not strongly influence raft-stabilizing ability nearly as much as changes in acyl chain length. The observation that the physical behavior of these analogues is similar to that of natural ceramides is confirmed by measurements of ceramide-induced displacement of sterol from rafts. We previously demonstrated that ceramide and chol compete to be accommodated in rafts, and that ceramide, which is preferentially accommodated, displaces sterols from the rafts [19]. Fig. 5 shows that displacement of DHE from rafts by ceramide analogues with a modified sphingoid base is very similar to that for C16:0-ceramide. It is interesting that the dipole potential of monolayers containing the C16:0-ceramide analogues have been previously found to be quite dependent on ceramide analogue structure [24]. Thus, our results indicate that dipole potential does not strongly influence raft formation.
The observation that small changes in ceramide structure do not greatly change the ability to stabilize rafts (with the exception of C16:0-D-ribophytoceramide, which stabilized rafts to a significantly greater degree than C16:0-ceramide), or disrupt the ability to displace chol from rafts is somewhat surprising because previous studies with chol analogues demonstrated that raft stability is sensitive to small perturbations in sterol structure [18, 32, 37]. It may be that changes in the ability of lipids to pack tightly is more sensitive to small changes in the molecular structure of their hydrophobic segments than small changes in their polar headgroups. This would be consistent with the observation that ceramide acyl chain length had a larger effect on ordered domain Tm than changes in ceramide headgroup structure. In most cases in which lipid headgroup structure is small, it may be possible for the lipid headgroup to adjust conformation to avoid interfering with lipid packing. The large ordered domaiN-stabilizing effect of phytoceramide may reflect additional hydrogen bonding capability.
The fact that long-chain ceramide analogues which are likely to resist normal metabolic breakdown pathways, can have raft formation properties similar to those of natural ceramides suggests that they may have useful applications in cellular and biomedical studies. C12:0-Ceramide analogues may be the best for such applications if they can be delivered into cells more easily than longer chain analogues (see below).
Use of short-chain vs. long-chain ceramide to study biological activity
Since the identification of ceramide as a lipid second messenger, numerous investigators have employed ceramide analogues to study the biological roles of ceramide [43–49]. Short-chain ceramides are frequently used because they are taken up into cells more easily than long-chain ceramides. However, our study and that of Nybond et al. [40] show that short-chain ceramides cannot support lipid raft formation as well as long-chain ceramides. This might alter the effects of short-chain ceramide analogues on some cellular processes. In fact, in rat basophilic leukemia cells, C2:0- and C6:0-ceramides have been reported to disrupt membrane order [50], consistent with raft-destabilizing behavior.
Thus, it is not surprising that there are differences in the biological actions of short- and long-chain ceramides. For example, the effects of ceramide on apoptosis have been extensively investigated. Short-chain ceramides induce apoptosis in several cancer cell lines [51–53], while longer-chain natural length ceramides are much less effective [54]. This difference may partly be an artifact of the difficulty of delivering long chain ceramides into cells [54]. However, while natural ceramides form large ceramide-rich raft platforms that are indispensable in Fas ligand-mediated apoptosis [13], short chain ceramides should not have this ability. This suggests that ceramide participates in more than one aspect of the apoptotic process. One way it may act is as a molecule that directly interacts and modulates the activity of proteins in the apoptotic signaling pathway. Forming ordered domains that act as signaling platforms to cluster membrane proteins involved in apoptosis may reflect a separate aspect of ceramide action. In addition, it is interesting to note that C2:0-ceramide, but not C16:0-ceramide, was able to cause loss of membrane potential in isolated mitochondria, suggesting that these short chain and long chain ceramides may accumulate in different locations [55]. It should also be noted that comparing studies in which chemical properties of ceramides were varied are complicated by differences in the experimental time scale, which can range from minutes to days. In some cases, direct actions of ceramide may be involved, while in other cases metabolites of ceramide may be involved, e.g. remodeling of short-chain ceramides to long-chain ceramides within cells. In any case, C12:0-ceramide and its derivatives may be useful for studies of ceramide action as they maintain the essential raft-stabilizing properties of natural ceramides, and might be easier to incorporate into cells than longer chain ceramides in some situations [54].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 2.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. UV-C light induces raft-associated acid sphingomyelinase and JNK activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Mattjus P, Schmid PC, Dong Z, Zhong S, Ma WY, Brown RE, Bode AM, Schmid HH, Dong Z. Involvement of the acid sphingomyelinase pathway in uva-induced apoptosis. J Biol Chem. 2001;276:11775–11782. doi: 10.1074/jbc.M006000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher LM, Wiegmann K, Futterer A, Pfeffer K, Machleidt T, Schutze S, Mak TW, Kronke M. CD28 signals through acidic sphingomyelinase. J Exp Med. 1995;181:2059–2068. doi: 10.1084/jem.181.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni HT, Deeths MJ, Li W, Mueller DL, Mescher MF. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J Immunol. 1999;162:5183–5189. [PubMed] [Google Scholar]
- 6.Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced "acidic" sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 8.Zhang DX, Yi FX, Zou AP, Li PL. Role of ceramide in TNF-alpha-induced impairment of endothelium-dependent vasorelaxation in coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H1785–1794. doi: 10.1152/ajpheart.00318.2002. [DOI] [PubMed] [Google Scholar]
- 9.Grassme H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, Harzer K, Lang F, Meyer TF. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 10.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 11.Grassme H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 12.Jan JT, Chatterjee S, Griffin DE. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol. 2000;74:6425–6432. doi: 10.1128/jvi.74.14.6425-6432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 14.Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.London E. Insights into lipid raft structure and formation from experiments in model membranes. Curr Opin Struct Biol. 2002;12:480–486. doi: 10.1016/s0959-440x(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah J, Atienza JM, Rawlings AV, Shipley GG. Physical properties of ceramides: effect of fatty acid hydroxylation. J Lipid Res. 1995;36:1945–1955. [PubMed] [Google Scholar]
- 18.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 19.Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Alterman M, Dobrowsky RT. Ceramide displaces cholesterol from lipid rafts and decreases the association of the cholesterol binding protein caveolin-1. J Lipid Res. 2005;46:1678–1691. doi: 10.1194/jlr.M500060-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Alanko SM, Halling KK, Maunula S, Slotte JP, Ramstedt B. Displacement of sterols from sterol/sphingomyelin domains in fluid bilayer membranes by competing molecules. Biochim Biophys Acta. 2005;1715:111–121. doi: 10.1016/j.bbamem.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 23.Megha, Bakht O, London E. Cholesterol precursors stabilize ordinary and ceramiderich ordered lipid domains (lipid rafts) to different degrees. Implications for the Bloch hypothesis and sterol biosynthesis disorders. J Biol Chem. 2006;281:21903–21913. doi: 10.1074/jbc.M600395200. [DOI] [PubMed] [Google Scholar]
- 24.Brockman HL, Momsen MM, Brown RE, He L, Chun J, Byun HS, Bittman R. The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys J. 2004;87:1722–1731. doi: 10.1529/biophysj.104.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun J, He L, Byun HS, Bittman R. Synthesis of ceramide analogues having the C(4)–C(5) bond of the long-chain base as part of an aromatic or heteroaromatic system. J Org Chem. 2000;65:7634–7640. doi: 10.1021/jo001227f. [DOI] [PubMed] [Google Scholar]
- 26.He L, Byun HS, Bittman R. A stereocontrolled, efficient synthetic route to bioactive sphingolipids: synthesis of phytosphingosine and phytoceramides from unsaturated ester precursors via cyclic sulfate intermediates. J Org Chem. 2000;65:7618–7626. doi: 10.1021/jo001225v. [DOI] [PubMed] [Google Scholar]
- 27.Sawatzki P, Kolter T. Syntheses of 3-C-Methylceramides. Eur. J. Org. Chem. 2004;2004:3693–3700. [Google Scholar]
- 28.Furukawa J, Kawabata N, Nishimura J. Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide. Tetrahedron. 1968;24:53–58. [Google Scholar]
- 29.S P, Brodesser S, Kolter T. Bioorganic synthesis of ceramide. Eur J Org Chem. 2003;2003:2021–2024. [Google Scholar]
- 30.Palomo C, Oiarbide M, Mielgo A. Unveiling reliable catalysts for the asymmetric nitroaldol (Henry) reaction. Angew Chem Int Ed Engl. 2004;43:5442–5444. doi: 10.1002/anie.200460506. [DOI] [PubMed] [Google Scholar]
- 31.Megha, London E, Bakht O. Cholesterol precursors stabilize ordinary and ceramiderich ordered lipid domains (lipid rafts) to different degrees: Implications for the Bloch hypothesis and sterol biosynthesis disorders. J Biol Chem. 2006 doi: 10.1074/jbc.M600395200. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 34.Recktenwald DJ, McConnell HM. Phase equilibria in binary mixtures of phosphatidylcholine and cholesterol. Biochemistry. 1981;20:4505–4510. doi: 10.1021/bi00518a042. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 36.Silvius JR, del Giudice D, Lafleur M. Cholesterol at different bilayer concentrations can promote or antagonize lateral segregation of phospholipids of differing acyl chain length. Biochemistry. 1996;35:15198–15208. doi: 10.1021/bi9615506. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Megha, London E. Relationship between sterol/steroid structure and participation in ordered lipid domains (lipid rafts): implications for lipid raft structure and function. Biochemistry. 2004;43:1010–1018. doi: 10.1021/bi035696y. [DOI] [PubMed] [Google Scholar]
- 38.Cevc G. How membrane chain-melting phase-transition temperature is affected by the lipid chain asymmetry and degree of unsaturation: an effective chain-length model. Biochemistry. 1991;30:7186–7193. doi: 10.1021/bi00243a021. [DOI] [PubMed] [Google Scholar]
- 39.Waarts BL, Bittman R, Wilschut J. Sphingolipid and cholesterol dependence of alphavirus membrane fusion. Lack of correlation with lipid raft formation in target liposomes. J Biol Chem. 2002;277:38141–38147. doi: 10.1074/jbc.M206998200. [DOI] [PubMed] [Google Scholar]
- 40.Nybond S, Bjorkqvist YJ, Ramstedt B, Slotte JP. Acyl chain length affects ceramide action on sterol/sphingomyelin-rich domains. Biochim Biophys Acta. 2005;1718:61–66. doi: 10.1016/j.bbamem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Duzgunes N, Wilschut J, Hong K, Fraley R, Perry C, Friend DS, James TL, Papahadjopoulos D. Physicochemical characterization of large unilamellar phospholipid vesicles prepared by reverse-phase evaporation. Biochim Biophys Acta. 1983;732:289–299. doi: 10.1016/0005-2736(83)90214-6. [DOI] [PubMed] [Google Scholar]
- 42.Beck A, Heissler D, Duportail G. Influence of the length of the spacer on the partitioning properties of amphiphilic fluorescent membrane probes. Chem Phys Lipids. 1993;66:135–142. doi: 10.1016/0009-3084(93)90038-5. [DOI] [PubMed] [Google Scholar]
- 43.Bielawska A, Linardic CM, Hannun YA. Modulation of cell growth and differentiation by ceramide. FEBS Lett. 1992;307:211–214. doi: 10.1016/0014-5793(92)80769-d. [DOI] [PubMed] [Google Scholar]
- 44.Chik CL, Li B, Karpinski E, Ho AK. Ceramide inhibits L-type calcium channel currents in GH3 cells. Mol Cell Endocrinol. 2004;218:175–183. doi: 10.1016/j.mce.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 45.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 46.Jin JS, Tsai CS, Si X, Webb RC. Endothelium dependent and independent relaxations induced by ceramide in vascular smooth muscles. Chin J Physiol. 1999;42:47–51. [PubMed] [Google Scholar]
- 47.Mathias S, Dressler KA, Kolesnick RN. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sjoholm A. Ceramide inhibits pancreatic beta-cell insulin production and mitogenesis and mimics the actions of interleukin-1 beta. FEBS Lett. 1995;367:283–286. doi: 10.1016/0014-5793(95)00470-t. [DOI] [PubMed] [Google Scholar]
- 49.Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270:22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 50.Gidwani A, Brown HA, Holowka D, Baird B. Disruption of lipid order by short-chain ceramides correlates with inhibition of phospholipase D and downstream signaling by FcepsilonRI. J Cell Sci. 2003;116:3177–3187. doi: 10.1242/jcs.00621. [DOI] [PubMed] [Google Scholar]
- 51.Ahn EH, Schroeder JJ. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp Biol Med (Maywood) 2002;227:345–353. doi: 10.1177/153537020222700507. [DOI] [PubMed] [Google Scholar]
- 52.Fillet M, Bentires-Alj M, Deregowski V, Greimers R, Gielen J, Piette J, Bours V, Merville MP. Mechanisms involved in exogenous C2- and C6-ceramide-induced cancer cell toxicity. Biochem Pharmacol. 2003;65:1633–1642. doi: 10.1016/s0006-2952(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 53.Takai N, Ueda T, Kawano Y, Nishida M, Nasu K, Narahara H. C2-ceramide exhibits antiproliferative activity and potently induces apoptosis in endometrial carcinoma. Oncol Rep. 2005;14:1287–1291. [PubMed] [Google Scholar]
- 54.Shabbits JA, Mayer LD. Intracellular delivery of ceramide lipids via liposomes enhances apoptosis in vitro. Biochim Biophys Acta. 2003;1612:98–106. doi: 10.1016/s0005-2736(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 55.Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+ -dependent or a Ca2+ -independent mechanism. J Bioenerg Biomembr. 2004;36:165–170. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]


