Abstract
Spermatogonial stem cells are required for the initiation of spermatogenesis and the continuous production of sperm. In addition, they can acquire pluripotency and differentiate into derivatives of the three embryonic germ layers when cultured in the appropriate conditions. Therefore, understanding the signaling pathways that lead to self-renewal or differentiation of these cells is of paramount importance for the treatment of infertility, the development of male contraceptives, the treatment of testicular cancers, and ultimately for tissue regeneration. In this report, we studied some of the signaling pathways triggered by glial cell line-derived neurotrophic factor (GDNF), a component of the spermatogonial stem cell niche produced by the somatic Sertoli cells. As model systems, we used primary cultures of mouse spermatogonial stem cells, a mouse spermatogonial stem cell line and freshly isolated testicular tubules. We report here that GDNF promotes spermatogonial stem cell proliferation through activation of members of the Src kinase family, and that these kinases exert their action through a PI3K/Akt-dependent pathway to up-regulate N-myc expression. Thus, to proliferate, spermatogonial stem cells activate mechanisms that are similar to the processes observed in brain stem cells and lung progenitors.
INTRODUCTION
Spermatogenesis is a complex cellular process that starts with the self-renewal and differentiation of a small population of spermatogonial stem cells, called Asingle spermatogonia. Understanding the biology of spermatogonial stem cells is of paramount importance to elucidate basic mechanisms of cell self-renewal versus differentiation, for the treatment of infertility, for the development of male contraceptives and to clarify the etiology of certain testicular cancers. In addition, differentiated spermatogonial stem cells could be used to produce transgenic animals, preserve rare breeding stock, and maintain endangered species. Recently, the potential of mouse neonatal and adult spermatogonial stem cells to acquire ES cell properties and to generate derivatives from the three embryonic germ layers has been discovered; if this is true of human spermatogonial stem cells, this technology may provide a potentially new source of material for cell-based therapy (Kanatsu-Shinohara et al., 2004; Guan et al., 2006). In the testes, spermatogonial stem cells are found in the basal part of the seminiferous epithelium, in contact with the basement membrane. They are also in close association with the nursing Sertoli cells, which produce the growth factors necessary to induce self-renewal and differentiation. The microenvironment provided by the Sertoli cells and the basement membrane is called the stem cell niche (Xie and Spradling, 2000; Ogawa et al., 2005). One component of the niche is glial cell line derived neurotrophic factor (GDNF), which is a growth factor produced by Sertoli cells. GDNF signals through a multicomponent receptor system also found in the nervous system, skin, whisker pad, kidney, stomach, and skeletal muscle (Trupp et al., 1995; Golden et al., 1999). GFRα-1 is a co-receptor linked to the outer layer of the plasma membrane by a GPI anchor, and is thus localized to lipid rafts. Binding of GDNF to GFRα-1 molecules triggers the recruitment of the Ret transmembrane receptor to the rafts, and the formation of a signaling complex. Subsequent activation of Ret induces tyrosine autophosphorylation and the intracellular relay of the signal. In the mouse seminiferous epithelium, GFRα-1 is exclusively expressed by Asingle spermatogonia, and possibly their direct progeny, the Apaired spermatogonia, while Ret is expressed by all premeiotic germ cells (Dettin et al., 2003; von Schonfeldt et al., 2004; Hofmann et al., 2005a; Buageaw et al., 2005). Mice over-expressing GDNF exhibit an increased proliferation of Asingle and some clusters of Apaired spermatogonia that ultimately leads to testicular tumors resembling human seminoma (Meng et al., 2000; Meng et al., 2001). Conversely, the testes of GDNF, Ret and GFRα-1 null mice show depletion of the spermatogonial stem cells (Naughton et al., 2005). In addition, recent studies have shown that GDNF also enhances spermatogonial stem cell proliferation and differentiation in vitro (Kanatsu-Shinohara et al., 2003; Kubota et al., 2004; Hofmann et al., 2005a).
GDNF is known for triggering the self-renewal of spermatogonial stem cells and maintaining the spermatogenic lineage in vitro (Kubota et al., 2004). GDNF is also a prerequisite for the induction, but not the maintenance, of the ES cell-like phenotype (Kanatsu-Shinohara et al., 2004; Guan et al., 2006). It is thus crucial to unravel the molecular events underlying GDNF signaling in isolated spermatogonial stem cells to ultimately use them for regenerative medicine, or to understand male infertility and testicular neoplasias. However, signaling pathways leading to stem cell self-renewal and differentiation are poorly understood. This is due to the fact that stem cell populations are small, they are difficult to specifically isolate, and their survival rate in culture is poor. This is especially the case for spermatogonial stem cells. In the past, isolation of populations of germ cells at distinct steps of spermatogenesis has been possible using the STAPUT method developed by A. Bellvé and M. Dym. (Bellvé et al., 1977; Dym et al., 1995). This technique allows the separation of type A spermatogonia from 6-day-old mouse pups with a purity of 90%. We recently coupled the STAPUT method with an immunomagnetic bead technique, which allowed us to separate only the type A spermatogonia that express the GFRα-1 receptor (Asingle and Apaired cells) (Hofmann et al., 2005a). By using 4 to 5-day-old pups we can now work with a cell population that consists exclusively of Asingle spermatogonia.
Recent investigations have shown that non-receptor tyrosine kinases belonging to the Src family are developmentally regulated in the mouse testis (Nishio et al., 1995; Gye et al., 2005). There is a large increase in the expression of p60Src during the first 2 weeks of post-natal development, which is due predominantly to the increase in Sertoli cells numbers. However, a small number of spermatogonia in the basal part of the seminiferous epithelium show an intense p60Src expression as well (Gye et al., 2005). Since Ret receptor autophosphorylation allows the docking and activation of Src in neurons (Encinas et al., 2004), we sought to elucidate whether Src activation and Src-dependent pathways were triggered by GDNF in mouse spermatogonial stem cells, rather than a Ras-dependent pathway.
In this study, we used three distinct model systems: isolated GFRα-1 positive germ cells from neonatal mice testes, isolated seminiferous tubules from neonatal and adult mice, and a recently established spermatogonial stem cell line, C18–4, that expresses the GFRα-1 and Ret receptors, and responds to GDNF by increasing its rate of proliferation (Hofmann et al., 2005b). The cell line also expresses the proteins Oct-4 (Pesce et al., 1998), Dazl (Reijo et al., 2000) and GCNA (Enders and May, 1994), as well as high mRNA levels for ngn3(Yoshida et al., 2004), piwil2 (Cox et al., 2000; Lee et al., 2006) and prame-like (Steinbach et al., 2002), indicating its stem cell origin. C-kit transcripts (Manova et al., 1990) were also detected, but not the Kit protein. The cell line does not differentiate beyond the stage of early type A spermatogonia, which is probably due to the fact that the cells were immortalized using the large T antigen gene, a viral oncogene. Our data shows that in all three models studied, GDNF acts on Asingle spermatogonia by activating specific members of the Src family of protein kinases. In addition, we used the selective Src family inhibitor SU6656 to show that Src kinases are indeed required for spermatogonial stem cell proliferation. Further, we show that Src kinases activate a PI3K/Akt signaling pathway and induce N-myc expression in these cells. Thus, like in neuronal stem cells and lung progenitor cells, N-myc seems to play a role essential for the proliferation of spermatogonial stem cells.
MATERIALS AND METHODS
Animals
Male Balb/c mice (adults and 4–5 day-old pups) were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA). All animal care procedures were carried out according to the National Research Council’s Guide for the Care and Use of Laboratory Animals, and the experimental protocols employed in this study were approved by the Animal Care and Use Committee of the University of Dayton.
Staput isolation of type A Spermatogonia
Type A spermatogonia were isolated from the testes of 4 to 5-day-old pups using the STAPUT method that utilizes gravity sedimentation on a 2%–4% BSA gradient (Bellvé et al., 1977; Dym et al., 1995). Immediately after the STAPUT isolation, the spermatogonia were counted and resuspended in 30 ml D-MEM culture medium, supplemented with 5% fetal calf serum (FCS), 1 mM sodium pyruvate, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin and 100 mM non-essential amino acids. All tissue culture reagents were purchased from Atlanta Biologicals, Atlanta, GA, or from Hyclone/Fisher Scientific, Pittsburgh, PA. The cell suspension was placed into a 15-cm diameter tissue culture dish and incubated for 2 hours at 34°C in order to eliminate residual adherent Sertoli cells (differential plating)(Dirami et al., 2001). A total of 60–80 male pups were used for each isolation experiment, yielding an average of 3 × 106 type A spermatogonia with a purity of 90%.
Isolation of GFRα-1-positive spermatogonia
Magnetic beads (Dynabeads) were prepared in advance according to the manufacturer (Dynal/Invitrogen, Carlsbad, CA). Briefly, streptavidin-coated magnetic beads were incubated for 1 hour at room temperature with a biotinylated rabbit anti-goat secondary antibody (Vector Laboratories, Burlingame, CA) at a concentration of 2.5 μg for 4 × 107 beads in 100 μl phosphate buffered saline (PBS) containing 0.1% BSA. After STAPUT isolation and differential plating, the spermatogonia were centrifuged and resuspended in 1 ml of tissue culture medium containing 10% Nu serum (BD Biosciences, San Jose, CA) instead of FCS. The cells were incubated overnight at 4°C with a 1:200 dilution of a goat anti-mouse antibody recognizing the carboxy terminus of the GFRα-1 receptor (Santa Cruz Biotechnology, Santa Cruz, CA). Cells were then washed 3 times with PBS and incubated for 1 hour on a shaker at room temperature with the magnetic beads coated with the secondary antibody (ratio = 10 beads per target cell). The number of target cells was estimated at approximately 15 × 104 cells for 60 pups (de Rooij and Russell, 2000). After incubation, the GFRα-1 positive cells (coated with the magnetic beads) and the GFRα-1 negative cells (the cells without beads) were separated using a MPC-L magnet (Dynal/Invitrogen, Carlsbad, CA), yielding an average of 5–7.5 × 104 GFRα-1-positive spermatogonia with a purity of 98%.
The C18–4 cell line
The C18–4 cell line was established by stably transfecting type A spermatogonia with the Large T antigen gene driven by the ecdysone promoter (Hofmann et al., 2005b). From the cultures, a cell clone was isolated that expresses the GFRα-1 and Ret receptors, as well as the germ line markers Oct3/4 (Pesce et al., 1998) and Dazl (Reijo et al., 2000). The cells were cultured in complete cell culture media, which consisted of D-MEM media supplemented with 1 mM sodium pyruvate, 2 mM L-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 100 mM non-essential amino acids and 10% FCS. All tissue culture reagents were purchased from Atlanta Biologicals, Norcross, GA, or Hyclone/Fisher Scientific, Pittsburgh, PA. Cells were plated in 100-mm tissue culture dishes or 24-wells plates, and cultured at 34°C and 5% CO2 in a humidified incubator. For cultures that required serum-starved conditions, FCS was replaced with 10 % synthetic Nu serum (BD Biosciences, San Jose, CA).
Isolation of neonatal and adult mouse testicular tubules
Testes were removed from 40 neonatal (5 day-old) or 10 adult mice (Balb/c) and decapsulated. The tubules were digested in D-MEM containing 1 mg/ml collagenase and 1 μg/ml DNase (Sigma, St Louis, MO) for 20 minutes at 34°C to remove the Leydig and peritubular cells, as well as the basement membrane, and expose the spermatogonial stem cells. The tubules were carefully washed in D-MEM and placed in minimal media overnight (D-MEM supplemented with 10% Nu serum, BD Biosciences, San Jose, CA) and treated the next day according to the protocols described in the following sections.
GFRα-1 and Ret Expression in the C18–4 Cells
The cells were seeded onto LabTek glass chambers (Fisher Scientific, Pittsburgh, PA), cultured until 70% confluency and fixed with ice-cold methanol. The cells were incubated at 37°C for one hour with either a goat anti-mouse Ret antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at dilutions of 1:100 to 1:500 or a goat anti-mouse GFRα-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at dilutions of 1:100 to 1:500. After washing with PBS, the cells were incubated with a rabbit anti-goat secondary antibody conjugated to FITC or a rabbit anti-goat secondary antibody conjugated with biotin (Vector Laboratories, Burlingame, CA). Binding of the biotin-conjugated antibody was revealed with the immunoperoxidase technique using an AEC kit (Vector Laboratories, Burlingame, CA), which gives a pink-red precipitate at the reaction sites. Fluorescent sample slides were mounted in ProLong Antifade Reagent (Molecular Probes/Invitrogen, Carlsbad, CA). Samples were visualized with a BH2 Olympus microscope equipped with a DP12 camera (B&B Microscopes, Pittsburgh, PA).
Proliferation Assays
A. Spermatogonial Stem Cells
GFRα-1-positive cells were isolated from 4 to 5-day-old mice testes as described above. They were seeded in 96-well microtiter plates at a concentration of 10,000 cells/well in 200 μl culture media. For baseline experiments, the cells were cultured either with D-MEM alone, or D-MEM and 10% Nu synthetic serum (BD Biosciences, San Jose, CA) or D-MEM, 10% Nu serum and 100 ng/ml GDNF (rabbit recombinant, R&D Systems, Minneapolis, MN). For cultures with pharmacological inhibitors, the media was complemented with 10% Nu serum and the samples treated with 100 ng/ml of GDNF (R&D Systems, Minneapolis, MN), or 100 ng/ml of GDNF and 10 μM of the Src inhibitor U6656 (Calbiochem, San Diego, CA), or 100 ng/ml of GDNF and 10 μM of the MEK inhibitor U0126 (Calbiochem, San Diego, CA), or 100 ng/ml of GDNF and 100 nM of the PIK3 inhibitor wortmannin (Sigma, St. Louis, MO), or 100 ng/ml of GDNF and 5 μg/ml of a GDNF neutralizing antibody (R&D Systems, Minneapolis, MN). The cells were cultured for 6 days and fresh treatment was applied daily. The number of cell clusters in each tissue culture well was counted on the sixth day. The data is presented as the average of triplicates (three separate experiments) plus or minus the standard deviation. ANOVA and Tukey’s post-hoc analysis were performed using SPSS to determine statistical significance.
B. C18–4 cell line
C18–4 cells were seeded at 10,000 cells per well in 24-wells culture plates with complete cell culture media and 10% Nu serum. Half of the wells were treated with 100 ng/ml GDNF (R&D Systems, Minneapolis, MN). The treatment was repeated daily for 5 days. Each day, 3 control and 3 GDNF-treated cultures were thoroughly trypsinized and cells counted with a hemocytometer. The data is presented as the average of triplicates, each triplicate from three separate experiments, plus or minus the standard deviation. The Student' t test was used for comparison of the data with or without GDNF for each day. To analyze growth in presence of pharmacological inhibitors, C18–4 cells were seeded at 10,000 cells per well in 24-wells culture plates with complete cell culture media and 10% Nu serum. Culture wells were treated with pharmacological inhibitors and the neutralizing antibody as described for the freshly isolated GFRα-1 positive spermatogonia. After 6 days of culture, the C18–4 cells were thoroughly trypsinized and counted with a hemocytometer. The data is presented as the average of triplicates from three separate experiments. ANOVA and Tukey’s post-hoc analysis were performed using SPSS to assess statistical significance.
Phospho-Src expression in spermatogonial stem cells
GFRα-1-positive cells were isolated from 4 to 5-day-old mice, seeded at a concentration of 1000 cells/well in a tissue culture-treated microtiter plate (Costar, Corning, NY), and serum-starved overnight in D-MEM with 10% Nu serum (minimal media). The following day, half of the wells were exposed to 100 ng/ml of GDNF for 4 hours. The cells were then fixed in ice-cold methanol and stained with a rabbit anti-mouse phospho-Src (Tyr416) primary antibody (Cell Signaling Technology, Beverly, MA). Binding of the antibody was revealed with a goat anti-rabbit secondary antibody coupled with biotin (Vector Laboratories, Burlingame, CA). Reaction sites were revealed using AEC kit (Vector Laboratories, Burlingame, CA). A minimum of 500 cells were counted in each well and the percentage of positive cells calculated. Results from three different experiments were averaged and the standard deviation calculated. The Student's t test was performed to assess statistical significance.
Src Kinase Activity
Freshly isolated GFRα-1-positive cells were serum-starved overnight, by culturing them in D-MEM with 10% Nu serum. The following day they were stimulated with 100 ng/ml of GDNF for 4 hours and proteins were isolated in a non-denaturing lysis buffer containing 10 mM Tris-HCl, pH 7.4, 50 mM NaCl, 2 mM MgCl2, 1 mM CaCl2, 1% Triton X-100, 1 mM Na3VO4, 50 mM NaF, 1mM PMSF and 1:100 protease inhibitors cocktail (all reagents purchased from Sigma, St Louis, MO). The C18–4 cell line was grown in complete cell culture media and 10% FCS until the cells were 75% confluent. The cells were then serum-starved overnight. The following morning the cells were stimulated with 100 ng/ml of GDNF for 4 hours and proteins isolated as described for the freshly isolated spermatogonia. Seminiferous tubules were isolated from the testes of 40 5-day-old pups, digested for 20 min with 1 mg/ml collagenase and 1μg/ml DNase (Sigma, St Louis, MO), washed and treated with GDNF as described above. Proteins were isolated using the same non-denaturing buffer. The three sets of proteins were then immunoprecipitated using a v-Src antibody that specifically recognizes p60SRC (EMD Biosciences, San Diego, CA). The immune complexes were washed three times with the non-denaturing lysis buffer, then twice with a Src kinase assay buffer containing 100mM Tris-HCl, pH 7.2, 125mM MgCl2, 25mM MnCl2, 2mM EGTA, 250 μM sodium orthovanadate, 2mM dithiothreitol (Upstate, Milford, MA). Src activity was assessed using an ELISA tyrosine kinase activity kit and the Src kinase assay buffer as recommended by the manufacturer (Chemicon International, Inc., Temecula, CA). Experiments were repeated with the addition of a GDNF-neutralizing antibody (R&D Systems, Minneapolis, MN) or with the Src inhibitor SU6656 (Calbiochem, San Diego, CA). A phosphopeptide standard curve was run in parallel, to determine the amount of phosphorylated substrate produced by the Src kinase. Results were expressed in ng phosphorylated substrate/mg of protein extract. Experiments were done in triplicates (3 different experiments) and the data presented as means +/− standard deviation. To compare data resulting from the different treatments and assess significance, ANOVA and Tukey’s post-hoc analysis were performed using SPSS.
Western blotting for phospho-Akt and phospho-Mek
Seminiferous tubules from 6-day-old mice were digested with collagenase to expose the spermatogonial stem cells as described above, and serum-starved overnight in D-MEM containing 10% Nu serum. The following morning, they were treated for 4 hours with 100 ng/ml GDNF (R&D Systems, Minneapolis, MN), 100 ng/ml GDNF + 10 μM of the Src inhibitor SU6656 (Calbiochem, San Diego, CA) or 100 ng/ml GDNF + 5 μg/ml neutralizing antibody (R&D Systems, Minneapolis, MN). The samples were then homogenized in the non-denaturing lysis buffer with the phosphatase inhibitors sodium orthovanadate (1 mM) and sodium fluoride (50 mM) as described above. The C18–4 cell line was treated and protein extracted using the same protocols. The samples were then concentrated using centrifugal concentrators from Millipore (cutoff = 30,000 Da) (Millipore, Danvers, MA). Protein concentrations were determined using the Bio-Rad DC assay (Bio-Rad Laboratories, Hercules, CA). Thirty μg of proteins/lane were run on a 7.5% Tris-HCl gel and transferred to a nitrocellulose membrane. The membranes were probed with a rabbit anti-mouse phospho-Akt (Ser 473) and a rabbit anti-mouse phospho-Mek antibody (Ser217/221) (Cell Signaling Technologies, Beverly, MA), each at a concentration of 1: 500 for 1.5 hours at room temperature, followed by an incubation with a horseradish peroxidase labeled anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 1: 5000 for 1.5 hours at room temperature. The proteins were then revealed using the SuperSignal® West Pico Chemiluminescent Substrate kit (Pierce, Rockford, IL). The phospho-Akt protein has a molecular weight of 60 kDa and the phospho-Mek protein has a molecular weight of 45 kDa. After detection, the membranes were stripped with the Restore™ Western Blot Stripping Buffer (Pierce, Rockford, IL), and re-probed with a rabbit anti mouse β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to normalize protein expression. The β-actin protein has a molecular weight of 42 kDa. The intensity of the protein bands was measured using the EpiChemi II (EC2) darkroom system from UVP Bioimaging Systems and the LabWorks 4.0 acquisition and analysis software (UVP, Inc., Upland, CA). Results are the averages of 4 separate experiments and are expressed as % of control plus or minus the standard deviation. An ANOVA and Tukey’s post-hoc analysis were performed using SPSS.
RNA interference assays
A. Src family kinases down-regulation
GFRα-1-positive spermatogonia were isolated as described above. They were seeded in 96-well microtiter plates at a concentration of 10 000 cells/well in 200 μl D-MEM media (HyClone/Fisher, Pittsburgh, PA). In all cultures, the media was complemented with 10% NU synthetic serum for a controlled environment (Fisher Scientific, Pittsburgh, PA). The cultures were transfected either with p60-src, c-yes, fyn, or lyn siRNAs (Santa Cruz Biotechnology, Santa Cruz, CA) using the RNAiFect kit (Qiagen, Valencia, CA). A scrambled siRNA sequence (Santa Cruz Biotechnology, Santa Cruz, CA) and non-transfected cells were used as controls. To assess the efficiency of RNA interference, the cells were collected 36 hours after siRNA treatment and total RNA isolated with the RNeasy mini kit (Qiagen, Valencia, CA). Genomic DNA contamination was eliminated by treating the samples with RQ1 RNase-free DNAse (1 U DNase/μg RNA) (Promega, Madison, WI) for 1 hour at 37°C. The reaction was stopped with a final concentration of 2 mM EGTA, pH 8.0, according to the manufacturer. The samples were further incubated at 65°C for 10 minutes to inactivate the DNase, after which the total RNA concentration was measured by spectrophotometry. The absence of genomic DNA in the samples was checked by regular PCR (no reverse-transcriptase) using actin primers followed by an agarose gel electrophoresis. For each sample, 50 ng RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and 2 μl cDNA were used in a nested PCR reaction using two primer sets for each of the genes (fyn, lyn, p60-src, c-yes, and β-actin), and the Invitrogen PCR SuperMix (Invitrogen, Carlsbad, CA). The primers were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used according to the manufacturer's protocols with minor changes. Briefly, the first PCR reaction was run using 2 μl of cDNA and the second PCR reaction was run using 6 μl of the first PCR reaction product. The PCR program was 94°C for 2 minutes, 30 cycles of 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 1 minute, followed by 72°C for 5 minutes. The PCR products were resolved on agarose gel electrophoresis, the gels stained for 30 min with SYBR green I fluorescent dye (Invitrogen, Carlsbad, CA) and the PCR products visualized and quantified with the EpiChemi II (EC2) darkroom system from UVP Bioimaging Systems and the LabWorks 4.0 acquisition and analysis software (UVP, Inc., Upland, CA). Semi-quantitative analysis was performed using actin expression in each sample as a standard. Results are the averages of three different experiments and are expressed as % of control plus or minus the standard deviation. An ANOVA and Tukey’s post-hoc analysis were performed using SPSS. For proliferation assays, the cells were transfected on day 0 and day 3 with either p60-src, c-yes, fyn, or lyn siRNAs (Santa Cruz Biotechnology, Santa Cruz, CA) using the RNAiFect kit (Qiagen, Valencia, CA). A scrambled siRNA sequence (Santa Cruz Biotechnology, Santa Cruz, CA) and non-transfected cells were used as controls. Half of the cultures were also treated with 100 ng/ml of GDNF (R&D Systems, Minneapolis, MN) and fresh treatments were applied daily. After 2 and 6 days, the number of cell clusters in each tissue culture well was counted. The data is presented as the average of triplicates collected in 2 separate experiments plus or minus the standard deviation. For each treatment, the Student's t test was used to assess the significance of data obtained in presence or absence of GDNF.
B. N-myc down-regulation
GFRα-1-positive spermatogonia and C18–4 cells were cultured as outlined above and transfected with N-myc siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) using the RNAiFect kit (Qiagen, Valencia, CA). Thirty-six hours after transfection, RNA was isolated and DNase treatment performed as described above. The efficiency of RNA interference was assessed by semi-quantitative RT-PCR, using the N-myc and β-actin primers for nested PCR from Santa Cruz Biotechnology, Santa Cruz, CA. The first PCR reaction was run using 2 μl of cDNA and the second PCR reaction was run using 3 μl of the first PCR reaction product. The PCR program was 94°C for 2 minutes, 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, followed by 72°C for 5 minutes. The PCR products were resolved on agarose gel electrophoresis, the gels stained for 30 min with SYBR green I fluorescent dye (Invitrogen, Carlsbad, CA) and N-myc expression quantified using β-actin expression as standard. For proliferation assays, the cultures were treated with 100 ng/ml of recombinant rat GDNF (R&D Systems, Minneapolis, MN) and transfected with N-myc siRNA at day 0 and day 3. Fresh GDNF treatments were applied daily. Cells transfected with a scrambled siRNA sequence (Santa Cruz Biotechnology, Santa Cruz, CA), non-transfected cells and cells cultured without GDNF were used as controls. After 6 days of culture, the number of spermatogonial clusters and the number of C18–4 cells were counted in each well. The data is presented as the average of triplicates plus or minus the standard deviation, obtained in 3 different experiments. For each treatment, the Student's t test was used to compare and assess the significance of the data in presence or absence of GDNF.
N-myc Expression Analysis using Real-Time PCR
Seminiferous tubules were isolated from 5-day-old testes, digested for 20 min with collagenase/DNase as described before, serum-starved overnight in minimal medium, washed and treated with 100 ng/ml GDNF (R&D Systems, Minneapolis, MN), or 100 ng/ml of GDNF and 10 μg/ml of a GDNF-neutralizing antibody (R&D Systems, Minneapolis, MN), or 100 ng/ml of GDNF and 10 μM of the Src inhibitor SU6656 (Calbiochem, San Diego, CA), or 100 ng/ml of GDNF and 10 μM of the MEK inhibitor U0126 (Calbiochem, San Diego, CA), or 100 ng/ml of GDNF and 100 nM of the PI3K inhibitor wortmannin (Sigma, St. Louis, MO). The following day total RNA was isolated from the tubules using TRIzol reagent (Sigma, St. Louis, MO). Genomic DNA contamination was eliminated by treating the samples with RQ1 RNase-free DNAse (1 U DNase/μg RNA) (Promega, Madison, WI) for 1 hour at 37°C as described in the previous section. The absence of genomic DNA in the samples was checked by regular PCR (no reverse-transcriptase) using actin primers followed by an agarose gel electrophoresis. One μg of RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Subsequent Real-Time PCR reactions used 2 μl of cDNA and 1X iQ SYBR Green Supermix from Bio-Rad (Bio-Rad, Hercules, CA) with 100 nM each of N-myc-specific primers. The primers used to detect mouse N-myc were 5′-ACT TCT ACT TCG GCG G-3′, Tm=47°C (forward), and 5′-TCT CCG TAG CCC AAT-3′, Tm= 44°C (reverse complement). The primers used to detect β-actin were 5′-GGA CTC CTA TGT GGG TGA CGA-3′, Tm = 59°C (forward) and 5′-GCC TCG GTG AGC AGC-3′, Tm = 52°C (reverse complement). The primers were designed and evaluated using the Net Primer program and ordered from Integrated DNA Technologies (IDT, Coralville, IA). Real-time PCR was performed using the iCycler iQ™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with the following cycling profile: cycle 1: 95°C for 3 minutes, cycle 2 was repeated 50 times: denaturing at 95°C for 30 seconds, annealing of primers at 45°C for 30 seconds, and elongation at 72°C for 1 minute and 50 seconds, cycle 3: 72°C for 1 minute. Negative controls were provided by the same reaction with omission of the reverse transcriptase. The iCycler software calculated the threshold cycle (CT), and the expression of N-myc mRNA relative to β-actin mRNA in each sample was calculated as ΔCT = CT(N-myc) − CT(β-actin). The sample that received no treatment was designated as the control and was used to calculate the relative expression of N-myc mRNA in the other samples by the comparative CT method (2−ΔΔCT), with ΔΔCT = [CT (N-myc) − CT(β-actin)]sample − [CT (N-myc) − CT(β-actin) ]control. Real-time PCR was repeated three times for each sample, in 3 different experiments, and mean values with standard error were used for comparison. Statistically significant differences between treatment groups were determined by an ANOVA and Tukey’s post-hoc analysis utilizing SPSS. N-myc expression was also evaluated by semi-quantitative RT-PCR using β-actin as a standard.
Nuclear expression of N-Myc
To detect a change in the nuclear expression of the N-Myc protein after stimulation with GDNF, the C18–4 cells and primary spermatogonial stem cells were plated in LabTek glass chambers (Fisher Scientific, Pittsburgh, PA) or a tissue culture-treated microtiter plate (Costar, Corning, NY) respectively, serum-starved overnight in D-MEM containing 10% Nu serum, then treated with 100 ng/ml GDNF for 3 days. Immunocytochemistry with a mouse monoclonal anti-N-Myc antibody (Abcam Inc, Cambridge, MA) at dilutions of 1:100 and 1:250 in PBS was performed as described above.
RESULTS
Spermatogonial stem cells express GFRα-1 and Ret, and respond to GDNF in vitro
We previously reported that mouse spermatogonial stem cells express the GFRalpha-1 and Ret receptors in the neonatal and the adult testis. Expression of the receptors was seen in vitro after magnetic bead isolation of the cells, and in vivo when the cells were kept in their environment in cultures of seminiferous tubules (Dettin et al., 2003; Hofmann et al., 2005a). In the present study, we isolated pure populations of GFRα-1-positive cells with the STAPUT/immunomagnetic bead method from 4.5 to 5-day old mice, and cultured them with the optimal concentration of 100 ng/ml GDNF (Hofmann et al., 2005a) in minimal media, e.g. with 10% synthetic serum (Nu serum) instead of FCS and without feeder layer to minimize the influence of other growth factors. In this condition, the cells proliferated, forming growing clusters during the first 6 days of culture as seen in Figure 1A. Few clusters formed when the cells were grown in minimal media without GDNF, and most cells remained isolated and eventually died (Figure 1B). To quantify proliferation, we measured the number of cells clusters obtained for 10,000 cells seeded after 6 days of culture with or without GDNF. Figure 1C shows a statistically significant increase in the number of cell clusters in presence of 100 ng/ml of GDNF in comparison to culture conditions in minimal media and D-MEM only. In general, the number and size of clusters increase over time, and when these formations contain more than 10 cells they are called colonies. Figure 2A and 2B show the expression of the GFRα-1 and Ret receptors at the surface of the C18–4 spermatogonial stem cell line. When 100 ng/ml GDNF is added to the C18–4 cells, there is also a statistically significant increase in cell numbers by day 3 after seeding, and the rate of proliferation markedly increases after 4 days of culture (Figure 2C).
Figure 1. Proliferation of GFRα-1 positive spermatogonia in vitro.
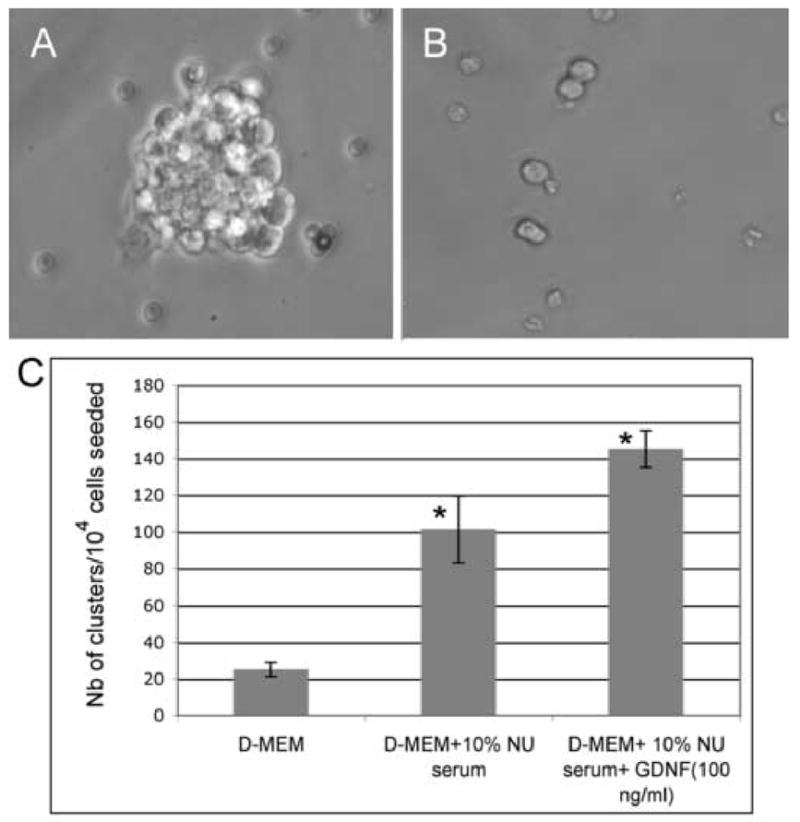
GFRα-1-positive spermatogonia were isolated using the Staput method followed by immunomagnetic bead separation. The cells were cultured in D-MEM alone, or in minimal media (D-MEM + 10% Nu serum), or in minimal media + 100 ng/ml GDNF. After 6 days, the number of growing cell clusters (5–10 cells) and colonies (>10 cells) was counted.
(A) Morphology of a colony in phase contrast microscopy after a 6-day treatment with 100 ng/ml GDNF in minimal media. (× 400).
(B) Morphology of a culture after 6 days without GDNF in minimal media (× 400). Most cells remain single or undergo only one or 2 divisions, producing small clusters.
(C) Number of cell clusters observed after 6 days of cultures in different conditions. The data is presented as the average of triplicates (3 separate experiments) plus or minus the standard deviation. Few small clusters (~3–4 cells) are observed when the cells are grown in D-MEM alone. The number of clusters significantly increases (*) in minimal media and in minimal media + 100 ng/ml GDNF (ANOVA, p <0.01). Furthermore, a Tukey's post-hoc test showed that each treatment was significantly different from the other two.
Figure 2. Expression of the GFRα-1 and Ret receptors at the surface of the C18–4 cells.
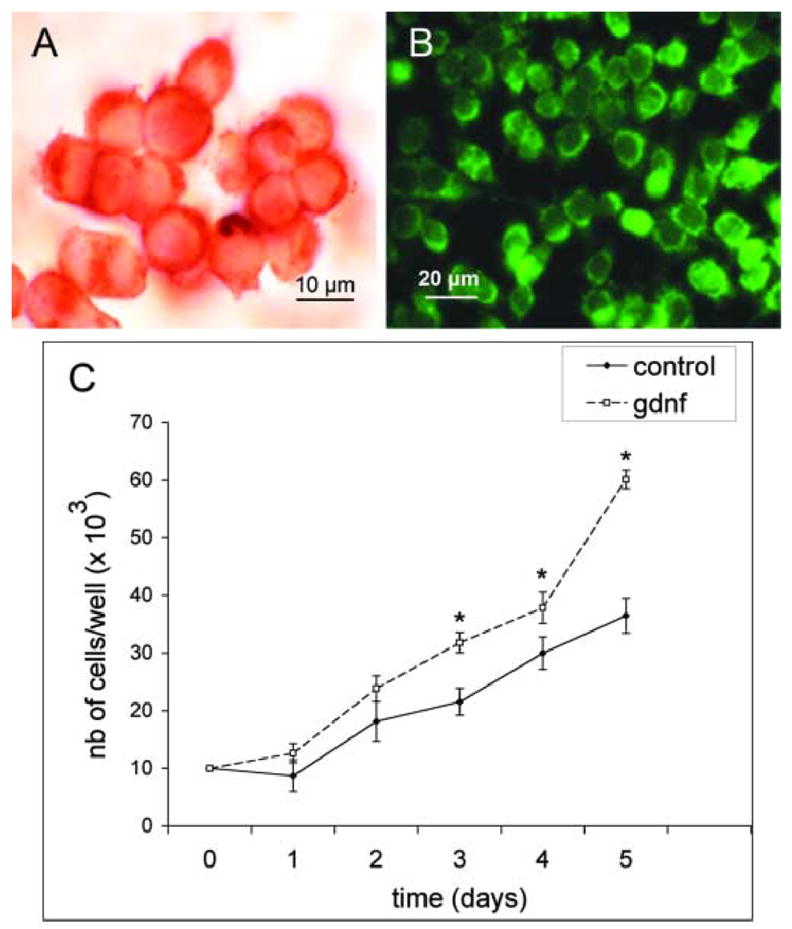
(A) C18–4 cells were cultured in 10% Nu serum until 80% confluency in LabTek chambers, fixed in ice-cold methanol and processed for immunocytochemistry for GFRα-1.
(B) Same as above, but the cells were processed for immunofluorescence for Ret.
(C) Growth curve of the C18–4 cell line showing an increase in the number of cells and their rate of proliferation in presence of GDNF. The data is shown as the average of triplicates, each triplicate from three separate experiments. The increase in cell numbers becomes significant by day 3 (*) and the rate of proliferation markedly increases after 4 days in culture. The Student's t test was used for comparison of the data with or without GDNF for each day (p<0.05).
GDNF induces Src kinase phosphorylation and activation in spermatogonial stem cells
We then treated freshly isolated GFRα-1-positive spermatogonia for 4 hours with 100 ng/ml GDNF, and probed the cells directly in the culture dishes for expression of phosphorylated Src at Tyr416 using immunocytochemistry. As seen in Figures 3A, isolated spermatogonial stem cells treated with GDNF did express phosphorylated Src at Tyr 416. In cultures that were not treated with GDNF but were stained for phosphorylated Src, most cells remained negative (Figure 3B). Moreover, the percentage of cells expressing phosphorylated Src increased significantly in comparison with the control (Figure 3C). We next measured Src kinase activity in isolated spermatogonial stem cells, the C18–4 cell line, and adult mouse seminiferous tubules using an ELISA-based kinase assay after immunoprecipitation using a v-Src antibody recognizing Src family members. In all 3 model systems, a 4-hours treatment with 100 ng/ml of GDNF significantly increased the level of Src kinase activity in comparison to the control cultures without GDNF (Figure 4). In addition, when 5 μg/ml of a GDNF-neutralizing antibody was used in conjunction with GDNF, no increase in kinase activity was observed, confirming that Src kinase activity is dependent on GDNF. Furthermore, when 10 μM of the Src-family inhibitor SU6656 was added to the cultures, no kinase activity was seen, further confirming the involvement of members of the Src kinase family in GDNF-induced signaling.
Figure 3. Src is phosphorylated in spermatogonial stem cells after GDNF induction.
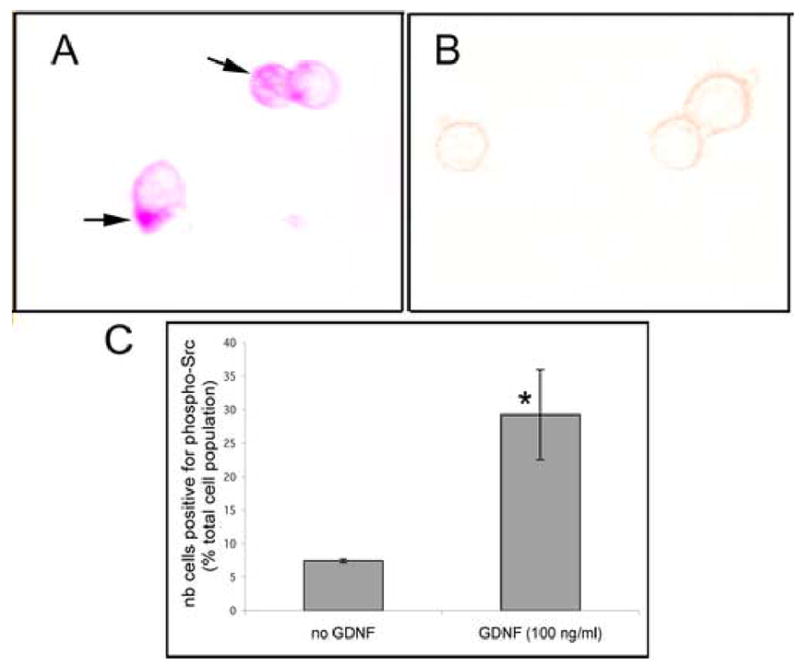
GFRα-1-positive cells were isolated using the STAPUT method followed by immunomagnetic bead separation. The cells were maintained overnight with 10% Nu serum in a microtiter plate and half of the cultures treated the next morning with 100 ng/ml GDNF for 4 hours. All cultures were then fixed directly in the culture wells and probed for the expression of P-Src at Tyr416 by immunocytochemistry. A minimum of 500 cells were counted in each well.
(A) Isolated GFRα-1 cells expressing phosphorylated Src (arrows) after GDNF-treatment.
(B) Isolated GFRα-1 cells that do not express phosphorylated Src (no GDNF treatment).
(C) Number of cells (% of total population) expressing phospho-Src in presence or absence of GDNF. The data represent the average of 3 separate experiments plus or minus the standard deviation. GDNF treatment results in a significant increase (*) in the number of cells positive for phosphorylated Src (Student's t-test, p value <0.05).
Figure 4. Src kinase activation by GDNF in spermatogonial stem cells.
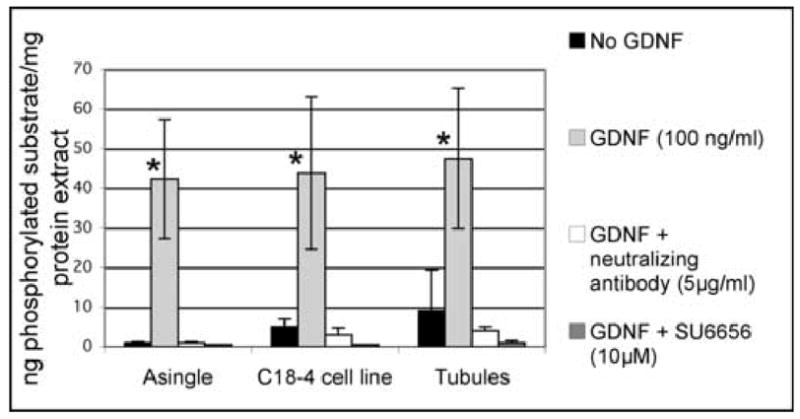
Freshly isolated GFRα-1 positive spermatogonia, the C18–4 cell line and stem cells in organ cultures were cultured overnight in minimal medium, then treated with 100 ng/ml GDNF, or GDNF and a GDNF-neutralizing antibody, or GDNF and the Src inhibitor SU6656. p60Src was immunoprecipitated and probed for phosphotyrosine activity using an ELISA-based assay. The data represent the average of 3 different experiments plus or minus the standard deviation. Without GDNF, very little enzyme activity could be detected. When 100 ng/ml GDNF was added, Src kinase activity increased significantly (*). Src kinase activity was abrogated when 5 μg/ml of a GDNF-neutralizing antibody was added to the GDNF cultures. Similarly, the kinase activity disappeared when 10 μM of SU6656 was added to the GDNF cultures, showing that this activity is indeed due to Src. (ANOVA, p value <0.01).
Src activation is necessary for spermatogonial stem cell proliferation
To determine if Src activation was necessary for spermatogonial stem cell proliferation, we cultured isolated spermatogonial stem cells and the C18–4 cells in presence of GDNF, a GDNF-neutralizing antibody and the Src inhibitor SU6656. As shown in Figure 5A, there is a significant increase in the number of clusters produced by isolated spermatogonial stem cells over a period of 6 days of culture with GDNF in comparison to the control cultures without the growth factor. This increase is not seen in the presence of GDNF together with a GDNF-neutralizing antibody, confirming that GDNF influences spermatogonial stem cell proliferation in vitro. In addition, the effects of GDNF are abolished when the cells are cultured with GDNF together with the Src inhibitor SU6656, suggesting that GDNF acts through a Src-activated pathway. Similarly, there is a significant increase in the number of C18–4 cells in presence of GDNF in comparison to the control cultures without GDNF. This effect is abolished by the GDNF-neutralizing antibody and the Src inhibitor SU6656 (Figure 5B).
Figure 5. Src kinase and PI3K activity are necessary for spermatogonial stem cell proliferation.
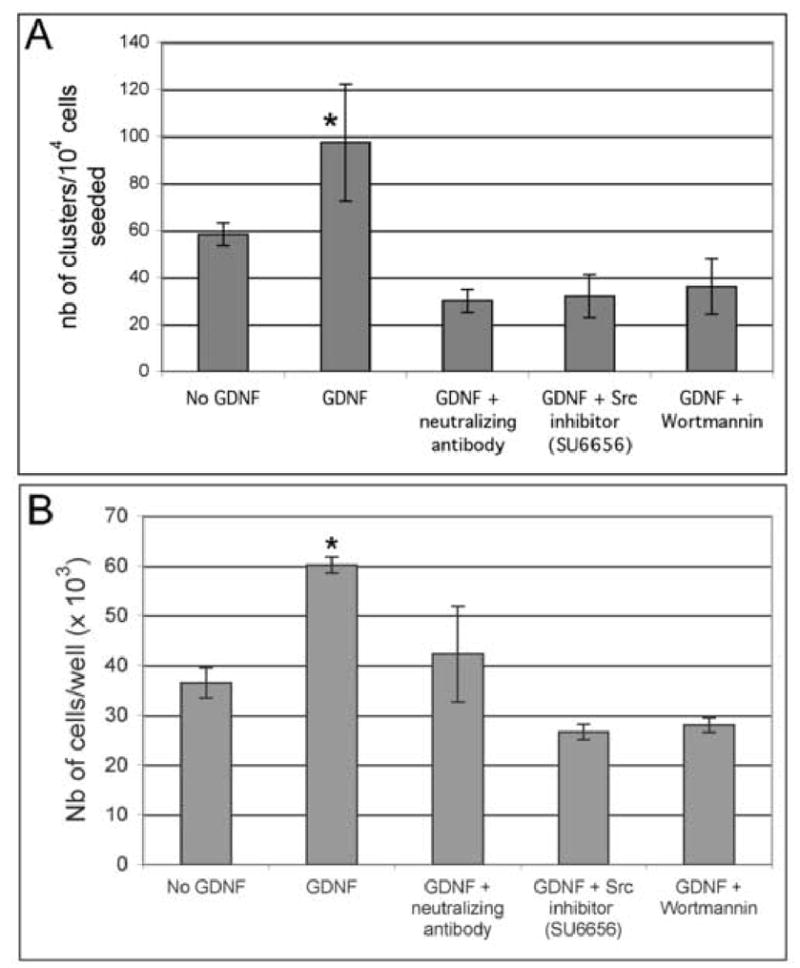
(A) GFRα-1-positive spermatogonia were isolated using the STAPUT method followed by immunomagnetic bead separation. The cells were cultured for 6 days in presence of 100 ng/ml GDNF, a GDNF-neutralizing antibody, and the Src inhibitor SU6656 or the PI3K inhibitor wortmannin. The data are presented as the average of 3 separate experiments plus or minus the standard deviation. In comparison with the control samples without GDNF, GDNF alone significantly increased the number of clusters (*). This increase is not observed when the cells are cultured in presence of GDNF and a GDNF-neutralizing antibody, or in presence of GDNF and pharmacological inhibitors such as SU6656 and wortmannin, suggesting that GDNF acts through a Src-PI3K pathway (ANOVA, p <0.01).
(B) Similarly, the C18–4 cells were cultured for 6 days in minimal media in the conditions described above. The data are presented as triplicates from 3 independent experiments plus or minus the standard deviation. GDNF alone significantly increased the number of C18–4 (*) cells while the increase is not observed in presence of GDNF and the pharmacological inhibitors (ANOVA, p <0.01).
In vertebrates, the Src family of tyrosine kinases contains 9 members, including p60-Src, Yes, Fgr, Yrk, Fyn, Lyn, Hck, Lck and Blk. Several members of the family are found in all tissues while others are more specialized (Thomas and Brugge, 1997). Therefore, we sought to identify if any member(s) of the Src family plays a preponderant role in spermatogonial proliferation. However, immunoprecipitation and western blotting are not particularly suitable to assess kinases involvement in isolated stem cells because these techniques need a fair amount of isolated proteins to obtain significant data. Therefore, we used RNA interference to test which Src kinases are involved in the proliferation of isolated stem cells. We attempted to down-regulate the expression of p60-src (c-src), c-yes, lyn and fyn, since only these kinases are affected by the Src inhibitor SU6656. Semi-quantitative RT-PCR analysis showed that the 4 kinases are indeed expressed in spermatogonia and that their mRNA expression was significantly reduced by the siRNA treatments compared with the scrambled siRNA controls or the no siRNA samples (Supplementary Figures 1A and 1B). Figure 6A shows that the early response to GDNF depends mainly on the Src family kinases p60-Src (c-Src) and c-Yes. After one week in culture, when spermatogonial proliferation is also linked to differentiation into Aaligned spermatogonia (Hofmann et al, 2005a), Lyn and Fyn also seem necessary (Figure 6B).
Figure 6. RNA interference analysis of the function of several Src family members.
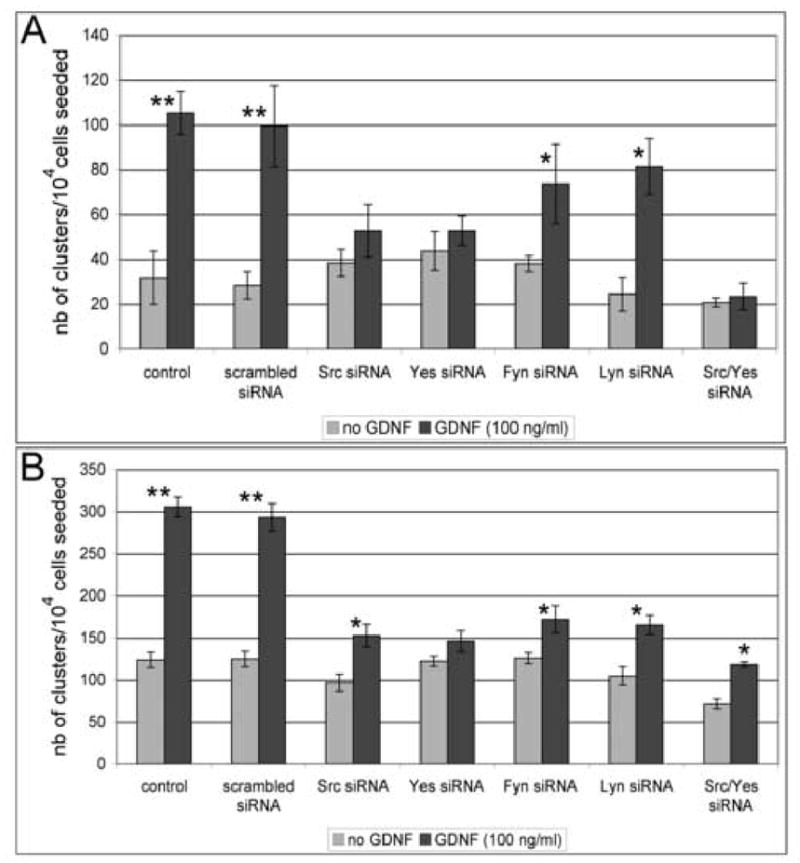
GFRα-1-positive spermatogonia were seeded in 96-well microtiter plates at a concentration of 10,000 cells/well in 200 μl D-MEM medium complemented with 10% NU synthetic serum to allow for a controlled environment. The cultures were transfected either with p60-src, c-yes, fyn, or lyn siRNAs. Non-transfected cells (control) and cells transfected with a scrambled siRNA sequence were used as references. The numbers of clusters obtained after 2 days and 6 days of culture were counted. The data are presented as triplicates, each from 2 independent experiments plus or minus the standard deviation. For each treatment, the Student's t test was used for comparisons between the cultures with and without GDNF.
(A) After two days of culture, GDNF significantly increases the number of clusters in non-transfected samples and samples transfected with a scrambled siRNA sequence (**=highly significant, p <0.005). The increase is also significant in cultures transfected with siRNAs for lyn and fyn (*= significant, p < 0.05). However, transfections with siRNAs for p60-src and c-yes abrogate the effects of GDNF, indicating that spermatogonial stem cell proliferation might depend on c-Src and c-Yes during the early response to GDNF.
(B) After 6 days of culture, GDNF significantly increases the number of clusters in non-transfected samples and samples transfected with a scrambled siRNA sequence (**= highly significant, p <0.005). The increase is also significant in cultures transfected with siRNAs for lyn, fyn, p60-src and c-yes (*= significant, p < 0.05). However, the number of clusters obtained after GDNF and siRNA treatments is markedly decreased when compared with the control cultures with GDNF alone (p <0.01), indicating that all kinases are involved in spermatogonial stem cell proliferation.
GDNF-dependent proliferation of spermatogonial stem cells also requires PI3K activity
The PI3K/Akt pathway has a pivotal role in GDNF-dependent neuron survival, neurite outgrowth and kidney bud development (Dudek et al., 1997; Jackson et al., 1996; Tang et al., 2002). To test whether PI3K activity was also necessary for GDNF-dependent proliferation of spermatogonial stem cells, we treated isolated spermatogonial stem cells and the C18–4 cells for 6 days with GDNF and wortmannin. As shown in Figure 5A and 5B, the effect of GDNF is drastically inhibited by addition of wortmannin in cluster cultures and in the C18–4 cell line, indicating that PI3K activity was also necessary for GDNF-induced proliferation of spermatogonial stem cells. To further investigate whether Src was promoting cell proliferation via a PI3K-dependent pathway or whether both PI3K and Src contributed to this biological effect via independent mechanisms, we treated spermatogonial stem cells in organ cultures and the C18–4 cell line with GDNF and SU6656, and investigated by western blotting whether or not Akt was phosphorylated. As seen in Figure 7, GDNF alone increased Akt phosphorylation, while treatment with GDNF and SU6656 decreased its expression. Taken together, the PI3K kinase/Akt pathway is necessary for GDNF-induced spermatogonial proliferation and requires Src activation.
Figure 7. The PI3K/Akt pathway is dependent on Src activation in spermatogonial stem cells.
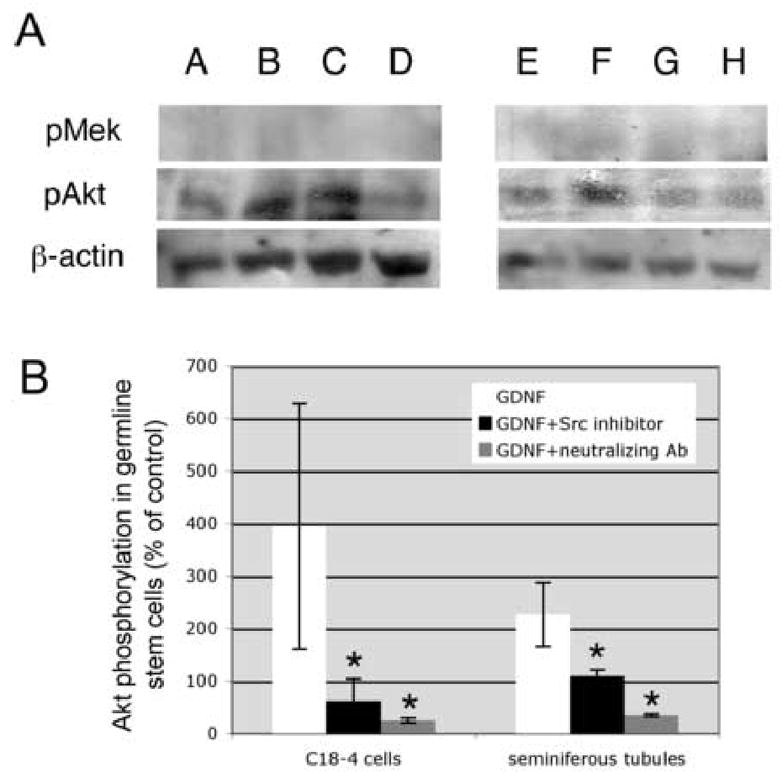
Spermatogonial stem cells in organ cultures and the C18–4 cell line were cultured overnight in minimal media, then treated for 4 hours with 100 ng/ml GDNF or 100 ng/ml GDNF and 10 μM SU6656. Total lysates (30 μg protein/lane) were resolved by PAGE and probed with specific antibodies against phospho-Akt and phospho-Mek. While GDNF induces Akt phosphorylation, a decrease in phospho-Akt is observed when the samples were treated with SU6656, indicating that the PI3K-Akt pathway is downstream of Src. Furthermore, no phospho-Mek is observed after GDNF treatment, indicating that a Ras-dependent pathway is not involved in these cultures.
(A) Western blot probed for pAkt, then stripped and re-probed for β-actin: Lane A= C18–4 cells control; lane B = C18–4 cells + 100 ng/ml GDNF; lane C = C18–4 cells + 100 ng/ml GDNF + SU6656; lane D = C18–4 cells + 100 ng/ml GDNF + neutralizing antibody (5 mg/ml); lane E = seminiferous tubules control; lane F = seminiferous tubules + 100 ng/ml GDNF; lane G = seminiferous tubules + 100 ng/ml GDNF + SU6656; lane H = seminiferous tubules + 100 ng/ml GDNF + neutralizing antibody (5 μg/ml).
(B) Graphic representation of the western blots after image analysis and normalizing over β-actin expression.
The data are presented as triplicates from 4 independent experiments plus or minus the standard deviation. There is a significant increase (*) in the levels of phosphorylated Akt after GDNF treatment (ANOVA, p <0.01).
Role of N-myc in GDNF-induced proliferation of spermatogonial stem cells
Our previous microarray analysis showed that genes important in cell proliferation and cell cycle regulation were differentially expressed by GFRα-1-positive spermatogonia when treated with GDNF (Hofmann et al., 2005a). In this condition, specific cyclins were upregulated while CDK inhibitors were downregulated, confirming that GDNF is activating the cell cycle and plays a role in spermatogonial stem cell proliferation. In addition, N-myc, a member of the myc proto-oncogene family, was upregulated by GDNF. The myc gene family is expressed in embryonic stem cells and the developing embryo, as well as in many adult stem cells (Cartwright et al., 2005; Satoh et al., 2004; Zanet et al., 2005). This gene family encodes transcription factors that are part of the early response to mitogenic signals. Among other functions, they can activate or repress transcription of cyclins and CDK inhibitors by interacting with specific binding partners and thus can impact a variety of cellular process that include stem cell proliferation, differentiation, and apoptosis (Bouchard et al., 2001; Staller et al., 2001; Eisenman, 2001). Further, the myc gene family is often activated by signaling pathways depending on Src activation (Bowman et al., 2001). Thus, this gene might play a central role in driving the proliferation of dividing spermatogonial stem cells. In Figure 8A we show by real-time PCR analysis that N-myc expression in spermatogonial stem cells (organ cultures) is up-regulated by GDNF. Moreover, N-myc expression is inhibited when the cells and testicular tubules are treated with the Src inhibitor SU6656 and wortmannin, indicating that N-myc expression is dependent on Src activation and a PI3K/Akt pathway. Another analysis using semi-quantitative RT-PCR showed the same effects, albeit not as prominent (Supplementary Figure 2). Further, we used RNA interference to demonstrate a biological effect of N-myc knockdown in isolated spermatogonial stem cells and the C18–4 cell line. Our data indicate that GDNF significantly increases the number of spermatogonial clusters in vitro, while this effect is suppressed when N-myc is knocked down (Figure 8B). Similarly, the proliferative effect of GDNF on the C18–4 cells is abolished when N-myc expression is inhibited (Figure 8C). Also, addition of GDNF markedly increased the nuclear expression of N-Myc in cultured spermatogonia stem cells and the C18–4 cell line, as seen by immunocytochemistry (Figure 8D). All C18–4 cells showed a weak basal nuclear expression of N-Myc (Figure 8D, b) that markedly increased in intensity after exposure to GDNF (Figure 8D, c). Interestingly, a dual pattern could be seen in clusters issued from primary spermatogonial stem cells, with only about half of the daughter cells staining strongly for N-Myc (Figure 8D, d). Taken together, N-myc plays a central role in GDNF-induced proliferation, but might be restricted to a subset of undifferentiated type A spermatogonia, possibly the spermatogonial stem cells.
Figure 8. GDNF induces spermatogonial stem cell proliferation through N-myc and a Src/PI3K-dependent pathway.
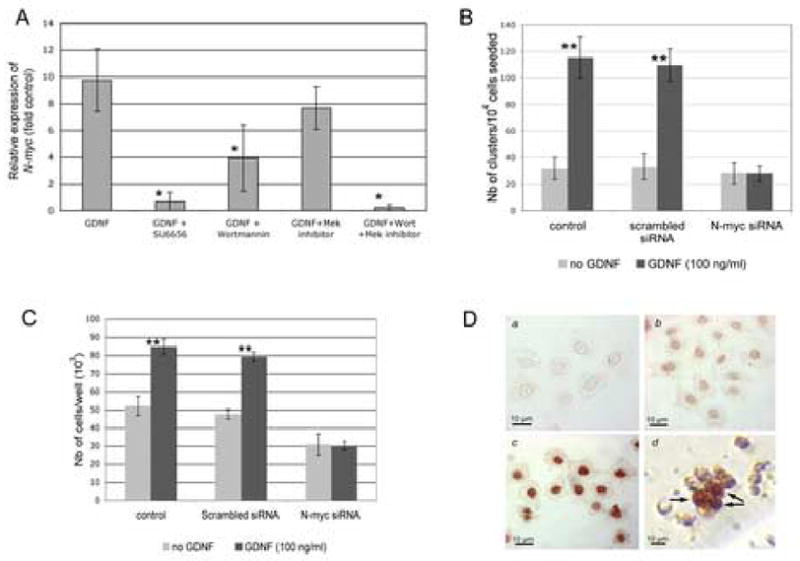
(A) Real-time PCR analysis of N-myc expression in presence of GDNF and pharmacological inhibitors of Src, PI3K and Mek. Spermatogonial stem cells in organ cultures were serum-starved overnight, then treated for 4 hours with 100 ng/ml GDNF, or 100 ng/ml GDNF and 10 μM SU6656, or 100 ng/ml GDNF and 100nM wortmannin, or 100 ng/ml GDNF and 10 μM of the Mek inhibitor U0126, or 100 ng/ml GDNF and 100nM wortmannin and 10 μM of U0126. Real-time PCR was repeated three times for each sample, in 3 separate experiments. N-myc expression was calculated as a relative intensity (fold control without GDNF) by the ΔΔCT method using actin as a standard. GDNF clearly up-regulates the expression of N-myc. However, N-myc expression is significantly decreased (*) in presence of GDNF and SU6656, or GDNF and wortmannin, but not in presence of GDNF and U0126. Thus, GDNF up-regulates N-myc expression through Src activation and PI3K signaling, and probably not through a Ras/Mek signaling pathway (ANOVA, p< 0.05).
(B) RNA interference study showing that N-myc is necessary for the proliferative action of GDNF in spermatogonial stem cells. GFRα-1-positive spermatogonia were seeded in 96-well microtiter plates at a concentration of 10,000 cells/well in 200 μl D-MEM medium complemented with 10% Nu synthetic serum to allow for a controlled environment. The cultures were transfected with N-myc siRNA. Non-transfected cells (control) and cells transfected with a scrambled siRNA sequence were used as references. The numbers of clusters obtained after 6 days of culture were counted. The data is presented as the average of 3 independent experiments plus or minus the standard deviation. For each treatment, the Student's t test was used for comparisons between the cultures with and without GDNF. GDNF significantly increases the number of clusters in non-transfected samples and samples transfected with a scrambled siRNA sequence (**=highly significant, p <0.005). However, transfections with siRNA for N-myc abrogate the effects of GDNF, indicating that GDNF needs N-Myc to induce spermatogonial stem cell proliferation.
(C) RNA interference study showing that N-myc is necessary for the proliferative action of GDNF in the C18–4 cells. C18–4 cells were seeded at a concentration of 104 cells in 24-wells plates and treated with the conditions described above. GDNF significantly increases cell proliferation in non-transfecrted samples and samples treated with scrambled siRNA (**= highly significant, p<0.005). However, transfections with N-myc siRNA abrogate the effects of GDNF, showing that GDNF uses N-Myc in the cell line to promote proliferation.
(D) Immunocytochemistry study showing the up-regulation of nuclear N-Myc expression after stimulation by GDNF. a: C18–4 cells with 10% Nu serum in the culture media, negative control (no first antibody); b: C18–4 cells with 10% Nu serum in the culture media, showing a weak, basal expression of N-Myc; c: C18–4 cells with 10% Nu serum and 100 ng/ml GDNF in the culture media, showing an increase in staining intensity for N-Myc in comparison to the basal expression; d: cluster of spermatogonial stem cells grown for 3 days in presence of GDNF (100 ng/ml) and showing strong up-regulation of N-myc only in some cells (arrows).
DISCUSSION
Recent investigations using in vitro cultures have confirmed that GDNF is a potent stimulator of spermatogonial stem cell self-renewal (Kanatsu-Shinohara et al., 2005). Elucidation of self-renewal and differentiation pathways that determine stem cell fate are of great interest for fundamental biology, and for clinical applications in reproductive medicine and cell-based therapy. Much effort is underway to determine cell intrinsic and microenvironmental signals that govern self-renewal and differentiation, and to identify genetic perturbations in these pathways that may result in tumorigenesis.
Using microarray analysis, we previously demonstrated that in these cells, GDNF alone was able to upregulate the expression of specific cyclins as well as N-myc, which is a member of the myc family found in brain and lung stem cells (Oliver et al., 2003; Okubo et al., 2005; Hofmann et al., 2005a). The present study was conducted to elucidate possible signaling pathways induced by GDNF and leading to the up-regulation of N-myc in spermatogonial stem cells, and to confirm its functional importance. To ensure controlled conditions, we obtained pure GFRα-1 positive spermatogonia and cultured them in minimal media in the presence of GDNF with or without inhibitors of specific kinases. In comparison, we also used spermatogonial stem cells in organ cultures (seminiferous tubules) and a recently established spermatogonial stem cell line. The results obtained with the three model systems are comparable. In this study, we have experimentally demonstrated that the proliferative effect of GDNF can be mediated by members of the Src family of non-receptor tyrosine kinases. This mechanism is thus similar to the situation described by Tansey et al. in neurons (Tansey et al., 2000). They and others also demonstrated that the activation of Src leads to activation of the Ras/Raf protein kinases or activation of the PI3/Akt signaling pathway, both of which will stimulate neuron survival (Besset et al., 2000; Encinas et al., 2001; Airaksinen and Saarma, 2002). Ret-mediated mitogenesis requires Src kinase activity in other cell types, and Src kinases have been shown to be over-expressed in a variety of cancers (Melillo et al., 1999; Tsygankov and Shore, 2004). In addition, the presence of p60-Src and Yes is necessary for the early response to GDNF by spermatogonial stem cells. These specific kinases are also important for embryonic stem cell self-renewal (Anneren et al., 2004), and our results confirm important similarities between spermatogonial stem cells and ES cells. Because signaling outside lipid rafts involves PI3K as well, but not Src, we investigated whether PI3K works downstream of Src or not (Paratcha et al., 2002). Since the phosphorylation of Akt, a target of PI3K, is abolished when SU6656 is added to the GDNF-stimulated cultures, we can assume that PI3K works downstream of Src. This correspond to the results of Encinas et al. with neuroblastoma cells transfected with a GFRα-1 construct (Encinas et al., 2001). However, we could not demonstrate any phosphorylation of the Mek kinase, at least at the Ser 217/221 residues. Because it is very difficult to quantify changes in protein expression in small populations of stem cells, it is possible that Mek phosphorylation was below detection level.
Further, we report for the first time that the GDNF-GFRα-1-Ret system induces the expression of N-myc via Src and PI3K activation. In addition to the quantitative assessment of N-myc RNA, we also detected an increase in N-Myc expression following GDNF exposure using immunocytochemistry. While the C18–4 cells showed a weak basal staining for N-Myc that markedly increased upon stimulation by GDNF, the response of the primary spermatogonial stem cells in culture was somewhat different, with only half of their daughter cells strongly expressing N-Myc after exposure to GDNF. Thus, these N-Myc-positive cells behave like the C18–4 immortalized cells and might be self-renewal spermatogonial stem cells. However, a true assessment of the pattern of expression of N-Myc (versus strict quantification) will depend on a better characterization of the cellular components of a growing colony, and will be the focus of further studies. A possible model is depicted in Figure 9.
Figure 9. GDNF/Src signaling in spermatogonial stem cells.
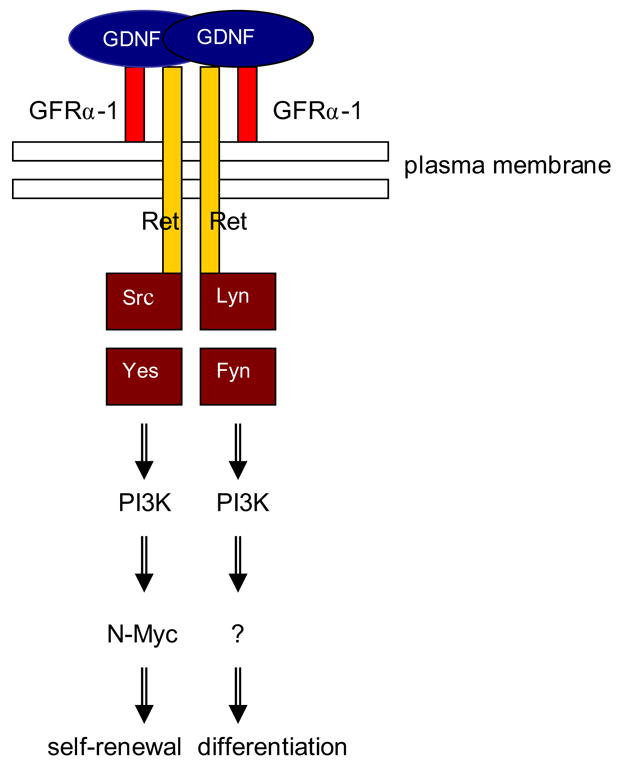
This model shows how GDNF can promote cell cycle progression via activation of Src kinase(s) and a PI3K/Akt pathway to increase N-myc gene expression. All 4 kinases depicted are inhibited by SU6656 and are involved in spermatogonial proliferation. p60-Src and c-Yes are necessary for the early response to GDNF, while the addition of Lyn and Fyn might be important for proliferation associated with differentiation. Because GDNF activates N-Myc expression in the spermatogonial stem cell line and a subpopulation of undifferentiated spermatogonia, it suggests that N-Myc activation is relevant for spermatogonial stem cell self-renewal only.
In contrast to the expression of the closely related c-myc gene, N-myc expression is very restricted in adult tissues. N-myc is expressed in normal neuronal precursors, where it promotes proliferation under the control of Sonic Hedgehog (Shh)(Oliver et al., 2003; Kenney et al., 2004). It is also expressed in the developing lung to maintain a distal population of undifferentiated, proliferating progenitor cells (Okubo et al., 2005). Not surprisingly, high levels of N-myc expression have been reported in human neuroblastoma and small cell lung carcinoma indicating that the cells at the origin of these tumors are possibly stem cells (Jakobovits et al., 1985). Moreover, N-myc overexpression has been shown in embryonal carcinoma and seminoma of the testis, indicating a possible involvement of undifferentiated germ cells in the etiology of these cancers (Saksela et al., 1989; Shuin et al., 1994). Studies performed by Meng and colleagues showed that transgenic mice over-expressing GDNF exhibited testicular tumors reminiscent to classical seminoma in men (Meng et al., 2001). In addition, Viglietto et al reported the expression of Ret and GFRα-1 in human testicular germ cell tumors, in particular seminomas (Viglietto et al., 2000). Therefore, the germ cell that is at the origin of seminoma might be a spermatogonial stem cell, and a deregulation of the system GDNF/GFRα-1/Ret could be part of the etiology of this type of cancer. However, many histological and immunocytochemistry studies indicate that gonocytes, the precursors of spermatogonial stem cells in the neonatal testis, are good candidates as well (Rajpert-De Meyts et al., 2003).
Like all stem cells, spermatogonial stem cells are characterized by two distinct responses to environmental cues: self-renewal or differentiation. An in depth analysis of the components of the spermatogonial stem cell niche will be necessary to understand these processes. This also includes the analysis of signaling pathways at the single cell level in response to a particular growth factor. Our study provides a first insight into the early response of spermatogonial stem cells to GDNF in vitro and identifies N-myc as a target of Ret signaling. Progress in unraveling cellular and molecular mechanisms including transduction and transcriptional events that guide self-renewal and differentiation will help devising new strategies for clinical intervention.
Supplementary Material
Semi-quantitative RT-PCR analysis of N-myc and Src family kinases expression in spermatogonial stem cells with or without siRNA treatments at 36 hours post-transfection. Beta-actin served as a standard. Data were obtained in triplicates from 3 separate experiments and are shown as the average plus and minus standard deviation. One-way ANOVA was run to determine the significance (p<0.05). Panel A: N-myc expression. Panel B: lyn expression. Panel C: fyn expression
Lane A: size markers; lane B: no siRNA; lane C: scrambled/control siRNA treatment; lane D: siRNA-1 treatment.
Semi-quantitative RT-PCR analysis of N-myc and Src family kinases expression in spermatogonial stem cells with or without siRNAs treatment at 36 hours post-transfection. Beta-actin served as a standard. Data were obtained in triplicates from 3 separate experiments and are shown as the average plus and minus standard deviation. One-way ANOVA was run to determine the significance (p<0.05). Panel D: c-yes expression. Panel E: p60-src expression.
Lane A: size markers; lane B: no siRNA; lane C: scrambled/control siRNA treatment; lane D: siRNA-1 treatment.
Semi-quantitative RT-PCR analysis of N-myc expression in spermatogonial stem cells (organ cultures) after treatments with GDNF, or GDNF and pharmacological inhibitors of Src, PI3K and Mek. Spermatogonial stem cells in organ cultures and the C18-4 cell line were cultured overnight in minimal media, then treated for 4 hours with 100 ng/ml GDNF, or 100 ng/ml GDNF and 10 μg/ml GDNF-neutralizing antibody (NA), or 100 ng/ml GDNF and 10 μM SU6656, or 100 ng/ml GDNF and 100nM wortmannin, or 100 ng/ml GDNF and 10 μM of the Mek inhibitor U0126. N-myc expression was standardized over beta-actin and expressed as a relative intensity (fold control sample without GDNF). The data are represented as the averages of 3 separate experiments plus or minus standard deviations. The GDNF treatment up-regulates N-myc expression. However, N-myc expression is significantly decreased (*) in presence of GDNF and the GDNF-neutralizing antibody, GDNF and SU6656, and GDNF and wortmannin, but not in presence of GDNF and U0126. Thus, GDNF up-regulates N-myc through Src activation and a PI3K-dependent pathway, but probably not through a Ras/Mek-dependent pathway (ANOVA, p< 0.05).
Acknowledgments
We would like to thank Meagan Roddy for valuable technical assistance and Dr. Sudhindra Gadagkar for his helpful comments on the statistical analysis. This work was funded by NIH grants HD36483 and HD044543, and a fellowship from the Consortium Research Fellows Program to L.B-S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen M, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Anneren C, Cowan C, Melton DA. The Src family of tyrosine kinases is important for embryonic stem cell self-renewal. J Biol Chem. 2004;279:31590–31598. doi: 10.1074/jbc.M403547200. [DOI] [PubMed] [Google Scholar]
- Bellve A, Cavicchia J, Millette C, O'brien D, Bhatnagar Y, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besset V, Scott R, Ibanez C. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem. 2000;275:39159–39166. doi: 10.1074/jbc.M006908200. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 2001;15:2042–2047. doi: 10.1101/gad.907901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger W, Sedivy J, Irby R, Yeatman T, Courtneidge S, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe V, Pholpramool C, Orwig K, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- Cartwright P, Mclean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Cox D, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Dettin L, Ravindranath N, Hofmann M, Dym M. Morphological Characterization of the Spermatogonial Subtypes in the Neonatal Mouse Testis. Biol Reprod. 2003;69:1565–1571. doi: 10.1095/biolreprod.103.016394. [DOI] [PubMed] [Google Scholar]
- Dirami G, Ravindranath N, Achi M, Dym M. Expression of Notch pathway components in spermatogonia and Sertoli cells of neonatal mice. J Androl. 2001;22:944–952. doi: 10.1002/j.1939-4640.2001.tb03434.x. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta S, Franke TF, Birnbaum M, Yao R, Cooper G, Segal R, Kaplan D, Greenberg M. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Dym M, Jia M, Dirami G, Price J, Rabin S, Mocchetti I, Ravindranath N. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod. 1995;52:8–19. doi: 10.1095/biolreprod52.1.8. [DOI] [PubMed] [Google Scholar]
- Eisenman R. Deconstructing Myc. Genes Dev. 2001;15:2023–2030. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- Encinas M, Crowder RJ, Milbrandt J, Johnson EJ. Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J Biol Chem. 2004;279:18262–18269. doi: 10.1074/jbc.M400505200. [DOI] [PubMed] [Google Scholar]
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EJ. c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G, May Jn. Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- Golden JP, Demaro J, Osborne P, Milbrandt J, Johnson EJ. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Guan K, Nayernia K, Maier L, Wagner S, Dressel R, Lee J, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006 doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- Gye M, Choi J, Ahn H, Kim Y. Postnatal changes in the expression of p60c-Src in mouse testes. Dev Growth Differ. 2005;47:233–242. doi: 10.1111/j.1440-169X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005a;279:114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dettin L, Johnson E, Dym M. Immortalization of mouse germ line stem cells. Stem Cells. 2005b;23:200–210. doi: 10.1634/stemcells.2003-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Blader IJ, Hammonds-Odie LP, Burga C, Cooke F, Hawkins P, Wolf A, Heldman KA, Theibert A. Initiation and maintenance of NGF-stimulated neurite outgrowth requires activation of a phosphoinositide 3-kinase. J Cell Sci. 1996;109:289–300. doi: 10.1242/jcs.109.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A, Schwab M, Bishop Jm, Martin G. Expression of N-myc in teratocarcinoma stem cells and mouse embryos. Nature. 1985;318:188–191. doi: 10.1038/318188a0. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, Toyoshima M, Niwa O, Oshimura M, Heike T, Nakahata T, Ishino F, Ogura A, Shinohara T. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kenney A, Widlund H, Rowitch DH. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development. 2004;131:217–228. doi: 10.1242/dev.00891. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock M, Brinster R. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73:173–179. doi: 10.1002/mrd.20391. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova R. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Melillo R, Barone M, Lupoli G, Cirafici A, Carlomagno F, Visconti R, Matoskova B, Di FPP, Vecchio G, Fusco A, Santoro M. Ret-mediated mitogenesis requires Src kinase activity. Cancer Res. 1999;59:1120–1126. [PubMed] [Google Scholar]
- Meng X, De Rooij DG, Westerdahl K, Saarma M, Sariola H. Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res. 2001;61:3267–3271. [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen M, Parvinen M, De Rooij DG, Hess M, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Naughton C, Jain S, Strickland A, Gupta A, Milbrandt J. Glial Cell-Line Derived Neurotrophic Factor (GDNF)-Mediated RET Signaling Regulates Spermatogonial Stem Cell Fate. Biol Reprod. 2005 doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Nishio H, Tokuda M, Itano T, Matsui H, Takeuchi Y, Hatase O. pp60c-src expression in rat spermatogenesis. Biochem Biophys Res Commun. 1995;206:502–510. doi: 10.1006/bbrc.1995.1072. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82:381–388. doi: 10.1532/IJH97.05088. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler P, Eisenman R, Hogan B. N-myc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Oliver T, Grasfeder L, Carroll A, Kaiser C, Gillingham C, Lin S, Wickramasinghe R, Scott M, Rj W-R. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100:7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF. Lipid rafts and the control of neurotrophic factor signaling in the nervous system: variations on a theme. Curr Opinion Neurobiol. 2002;12:542–549. doi: 10.1016/s0959-4388(02)00363-x. [DOI] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth D, Scholer H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev. 1998;71:89–98. doi: 10.1016/s0925-4773(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Rajpert-De Meyts E, Bartkova J, Samson M, Hoei-Hansen CE, Frydelund-Larsen L, Bartek J, Skakkebaek N. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS. 2003;111:267–78. doi: 10.1034/j.1600-0463.2003.11101301.x. discussion 278–9. [DOI] [PubMed] [Google Scholar]
- Reijo R, Dorfman D, Slee R, Renshaw A, Loughlin K, Cooke H, Page D. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol Reprod. 2000;63:1490–1496. doi: 10.1095/biolreprod63.5.1490. [DOI] [PubMed] [Google Scholar]
- Saksela K, Makela TPKA. Oncogene expression in small-cell lung cancer cell lines and a testicular germ-cell tumor: activation of the N-myc gene and decreased RB mRNA. Int J Cancer. 1989;44:182–185. doi: 10.1002/ijc.2910440132. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Matsumura I, Tanaka H, Ezoe S, Sugahara H, Mizuki M, Shibayama H, Ishiko E, Ishiko J, Nakajima K, Kanakura Y. Roles for c-Myc in self-renewal of hematopoietic stem cells. J Biol Chem. 2004;279:24986–24993. doi: 10.1074/jbc.M400407200. [DOI] [PubMed] [Google Scholar]
- Shuin T, Misaki H, Kubota Y, Yao M, Hosaka M. Differential expression of protooncogenes in human germ cell tumors of the testis. Cancer. 1994;73:1721–1727. doi: 10.1002/1097-0142(19940315)73:6<1721::aid-cncr2820730628>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Staller P, Peukert K, Kiermaier A, Seoane J, Lukas J, Karsunky H, Möröy T, Bartek J, Massague J, Hänel F, Eilers M. Repression of p15INK4B expression by Myc through association with Miz-1. Nat Cell Biol. 2001;3:392–399. doi: 10.1038/35070076. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Hermann J, Viehmann S, Zintl F, Gruhn B. Clinical implications of PRAME gene expression in childhood acute myeloid leukemia. Cancer Genet Cytogenet. 2002;133:118–123. doi: 10.1016/s0165-4608(01)00570-2. [DOI] [PubMed] [Google Scholar]
- Tang M, Cai Y, Tsai S, Wang Y, Dressler G. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Baloh R, Milbrandt J, Johnson EJ. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Thomas S, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez C. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankov A, Shore S. Src: regulation, role in human carcinogenesis and pharmacological inhibitors. Curr Pharm Des. 2004;10:1745–1756. doi: 10.2174/1381612043384457. [DOI] [PubMed] [Google Scholar]
- Viglietto G, Dolci S, Bruni P, Baldassarre G, Chiariotti L, Melillo RM, Salvatore G, Chiappetta G, Sferratore F, Fusco A, Santoro M. Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int J Oncol. 2000;16:689–694. doi: 10.3892/ijo.16.4.689. [DOI] [PubMed] [Google Scholar]
- Von Schonfeldt V, Wistuba J, Schlatt S. Notch-1, c-kit and GFRalpha-1 are developmentally regulated markers for premeiotic germ cells. Cytogenet Genome Res. 2004;105:235–239. doi: 10.1159/000078194. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling A. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takakura A, Ohbo K, Abe K, Wakabayashi J, Yamamoto M, Suda T, Nabeshima Y. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Zanet J, Pibre S, Jacquet C, Ramirez A, De Alboran IM, Gandarillas A. Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J Cell Sci. 2005;118:1693–1704. doi: 10.1242/jcs.02298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Semi-quantitative RT-PCR analysis of N-myc and Src family kinases expression in spermatogonial stem cells with or without siRNA treatments at 36 hours post-transfection. Beta-actin served as a standard. Data were obtained in triplicates from 3 separate experiments and are shown as the average plus and minus standard deviation. One-way ANOVA was run to determine the significance (p<0.05). Panel A: N-myc expression. Panel B: lyn expression. Panel C: fyn expression
Lane A: size markers; lane B: no siRNA; lane C: scrambled/control siRNA treatment; lane D: siRNA-1 treatment.
Semi-quantitative RT-PCR analysis of N-myc and Src family kinases expression in spermatogonial stem cells with or without siRNAs treatment at 36 hours post-transfection. Beta-actin served as a standard. Data were obtained in triplicates from 3 separate experiments and are shown as the average plus and minus standard deviation. One-way ANOVA was run to determine the significance (p<0.05). Panel D: c-yes expression. Panel E: p60-src expression.
Lane A: size markers; lane B: no siRNA; lane C: scrambled/control siRNA treatment; lane D: siRNA-1 treatment.
Semi-quantitative RT-PCR analysis of N-myc expression in spermatogonial stem cells (organ cultures) after treatments with GDNF, or GDNF and pharmacological inhibitors of Src, PI3K and Mek. Spermatogonial stem cells in organ cultures and the C18-4 cell line were cultured overnight in minimal media, then treated for 4 hours with 100 ng/ml GDNF, or 100 ng/ml GDNF and 10 μg/ml GDNF-neutralizing antibody (NA), or 100 ng/ml GDNF and 10 μM SU6656, or 100 ng/ml GDNF and 100nM wortmannin, or 100 ng/ml GDNF and 10 μM of the Mek inhibitor U0126. N-myc expression was standardized over beta-actin and expressed as a relative intensity (fold control sample without GDNF). The data are represented as the averages of 3 separate experiments plus or minus standard deviations. The GDNF treatment up-regulates N-myc expression. However, N-myc expression is significantly decreased (*) in presence of GDNF and the GDNF-neutralizing antibody, GDNF and SU6656, and GDNF and wortmannin, but not in presence of GDNF and U0126. Thus, GDNF up-regulates N-myc through Src activation and a PI3K-dependent pathway, but probably not through a Ras/Mek-dependent pathway (ANOVA, p< 0.05).


