Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is a disease with world wide consequences, affecting nearly a third of the world’s population. The established vaccine for TB, an attenuated strain of Mycobacterium bovis Calmette Guerin (BCG), has existed since 1921. Lactoferrin, an iron binding protein found in mucosal secretions and granules of neutrophils was hypothesized to be an ideal adjuvant to enhance the efficacy of the BCG vaccine, specifically because of previous reports of lactoferrin enhancement of IL-12 production from macrophages infected with BCG. Different vaccination protocols were investigated for generation of host protective responses against MTB infection using lactoferrin admixed to the BCG vaccine. Resulting effects demonstrate that BCG/lactoferrin increased host protection against MTB infection by decreasing organ bacterial load and reducing lung histopathology; significant reduction in tissue CFUs and pathology were observed post challenge compared to those seen with BCG alone. Addition of lactoferrin to the vaccine led to reduced pathological damage upon subsequent infection with virulent MTB, with positive results demonstrated when admixed in oil-based vehicle (incomplete Freund’s adjuvant; IFA) or when given with BCG in saline. The observed post-challenge results paralleled increasing production of IFN-γ and IL-6, but only limited changes to proinflammatory mediators TNF-α or IL-1β from BCG stimulated splenocytes. Overall, these studies indicate that lactoferrin is a useful and effective adjuvant to improve efficacy of the BCG vaccine, with potential to reduce related tissue damage and pulmonary histopathology.
Keywords: Lactoferrin, BCG, tuberculosis, vaccine adjuvant, immunization
2. Introduction
The Mycobacterium bovis bacillus Calmette Guerin (BCG), an attenuated strain of M. bovis, is the current vaccine for tuberculosis (TB), a disease caused by the intracellular pathogen Mycobacterium tuberculosis (MTB). BCG is the most widely used vaccine in the world and has remained relatively unchanged since 1921 [1, 2]. The World Health Organization (WHO) estimates that roughly one third of the world’s population is currently infected with MTB, and the incidence rate for new infections is approximately 0.6% per year [3]. While BCG is effective in protecting children against TB disease manifestations [4], the response wanes in adulthood, potentially leading to disease development upon infection [5]. The observed diminished efficacy of BCG vaccination has resulted in intensive research into developing novel TB vaccines that could surpass the efficacy of the current BCG vaccine [6–8]. Despite investigations into developing alternatives, BCG remains the gold standard for TB vaccines [9–11].
The BCG vaccine generates host protective responses against MTB infection by promoting development of a mycobacterial antigenic specific delayed type hypersensitivity (DTH) response, characterized as a T-cell helper type-1 (TH1) immunity with antigen specific production of IFN-γ [12]. A proven experimental method for enhancing the efficacy of the existing BCG vaccine is through addition of adjuvant components that lead to host protection against MTB infection, resulting in decreased organ bacterial load, reduced lung inflammation, and increased mycobacterial antigen specific production of IFN-γ [13–15].
A novel adjuvant candidate that could potentially enhance efficacy of the BCG vaccine is lactoferrin, an iron binding protein found primarily in mucosal secretions and neutrophilic granules [16]. Lactoferrin possesses a wide range of immunomodulatory activities [17–19] [20] [21] including promotion of the T-cell dominated DTH response towards BCG antigens [22, 23], The ability of lactoferrin to enhance the generation of antigen specific DTH responses suggests that lactoferrin could promote development of specific T-cell responses against a complex antigen, such as BCG [24, 25]
The development of T-cell helper type 1 (TH1) immunity is, in part, regulated by production of IL-12 [26, 27]. A variety of in vivo studies have shown lactoferrin capable of increasing production of IL-12 [28–30]. IL-12 is clearly a critical component involved in directing TH1 development effective in promoting protective host responses during MTB infection [31, 32]. More so, addition of lactoferrin to BCG infected macrophages increased the IL-12:IL-10 ratio, setting up an environment that could potentially promote BCG specific TH1 development in vivo [25, 33]. It was therefore hypothesized that lactoferrin possesses adjuvant activity that could be useful to enhance the efficacy of the BCG vaccine, leading to an increase in host protection against subsequent infection with MTB.
These studies were designed to investigate various vaccine formulations of BCG admixed with lactoferrin and the effect of different vaccine delivery schedules to increase host protection against subsequent infection with virulent Erdman MTB and to generate DTH responses to BCG antigens. The overall goal was to maximize the lactoferrin-mediated augmentation of the BCG vaccine, to result in decreased organ bacterial load and reduced lung histopathology following challenge with virulent mycobacterium. In addition, splenic recall to BCG antigens were examined to monitor for changes in production of IFN-γ to assess the adjuvant activity of lactoferrin to stimulate generation of cell mediated (TH1) immunity [34, 35].
3. Materials and Methods
3.1. Animals
Female C57BL/6 mice (6 weeks, Jackson Laboratories, Bar Harbor, ME) 20–25 g initial body weight, were used for splenocyte isolation. All in vivo experiments were conducted under approved guidelines of the animal ethics committee at the University of Texas, Health Science Center at Houston (HSC-AWC-03-106).
3.2. Lactoferrin and BCG
Low endotoxin bovine milk lactoferrin (< 1E.U./mg, < 20% iron saturated, >95% purity) was provided by PharmaReview Corporation (Houston, TX). Mycobacterium bovis Calmette Guerin (BCG), Pasteur strain, (TMC 1011, ATCC, Manassas, VA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37°C for 2 weeks before use. BCG was diluted with 1x Dulbecco’s phosphate-buffered saline (PBS) (Cellgro, Herndon, VA) to 3×108 organisms/mL, the concentration was estimated using McFarland standards (Sigma), and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS). Plates were incubated at 37°C with 5% CO2 for 3–4 weeks, and resulting colonies counted. Heat-killed BCG (HK-BCG) was achieved by autoclaving BCG suspension in 1x PBS at 121°C for 20min. Confirmation of heat-killed BCG was verified by plating on 7H11 plates and observing no growth of colonies after 3 weeks.
3.3. Vaccination
C57BL/6 female mice (8–10 weeks) were immunized with 100µL of vaccine formulation (up to 10 mice/group), subcutaneously, at the base of the tail. BCG was given at 1×106 – 1×107 CFU/mouse, and lactoferrin was given at 100µg/mouse. For the emulsion formulation, BCG with or without lactoferrin was emulsified with incomplete Freund’s adjuvant (IFA) (Difco, Detroit, MI) in a 1:1 (v/v) ratio. For the saline formulation, BCG with or without lactoferrin was combined in 1x PBS (“saline”). Mice were vaccinated a single time; a group of mice were boosted at indicated times post initial immunization. Experiments were repeated at least two times, using 6 or more mice/group per sacrifice time point.
3.4. Splenic recall
Splenocytes were isolated from immunized and non-immunized C57BL/6 mice at 6 weeks post the last immunization as previously described [25]. Briefly, spleens were minced, and red blood cells lysed with ACK lysing buffer (Cambrex Bio Sciences, East Rutherford, NJ). Splenocytes were cultured at 2×106 cells/mL in Dulbecco’s Modified Eagles Medium (DMEM, Sigma, St. Louis, MO) supplemented with 2.2g/L sodium bicarbonate, 0.05g/L of HEPES (Sigma), 0.05g/L L-arginine (Sigma), 100 µg/mL penicillin G (Sigma), 50 µg/mL gentamycin sulfate (Sigma) 0.005% 2-mercaptoethanol (2-Me, Gibco, Carlsbad, CA), and 10% fetal bovine serum (FBS, Sigma). Assay controls were performed to assess potential for cellular activation, including stimulation with lactoferrin (1–100 µg/mL), or with HK-BCG at multiplicity of infection (MOI) 10:1 (organisms:cells). Supernatants were collected at 24 and 72 hours post incubation and stored at −20°C for later cytokine analysis by ELISA.
3.5. Aerosol infection
Two or four weeks post-immunization, mice (6–10 mice/group) were aerosol challenged with Erdman strain of Mycobacterium tuberculosis (TMC 107, ATCC). MTB was grown in Dubos base with 5.6% glycerol and 10% supplement for 3–4 weeks before use. Bacteria were taken during log phase growth, resuspended in 1x PBS, sonicated for 10 seconds to dislodge any clumping that might have occurred. Organisms were diluted in 1x PBS and aerosolized using an inhalation exposure system (IES) (GLAS-COL Model #A4212 099c Serial #377782) [36]. Inoculation dose (approximately 100 CFU) was calculated by sacrificing 4 mice at day 1 post-infection and assessing for lung bacterial load by plating lung homogenates on 7H11 plates. Plates were incubated at 37°C with 5% CO2 for 3–4 weeks, and colonies were enumerated. Groups of mice were sacrificed at 7, 28, and 65 days post-challenge, and lung and spleen tissue was sectioned to evaluate bacterial load colony forming units (CFU), histology, and IFN-γ and TNF-α mRNA. Lung weight index (LWI), a rough measure of inflammation, was calculated as follows [37].
3.6. ELISA (Enzyme linked immunoadsorbant assay)
Supernatants from cultured splenocytes were assayed for cytokine production using the DuoSet ELISA kits (R&D Systems, Minneapolis, MN), according to manufacturer’s instructions. Splenocytes were assayed for production of T-cell cytokines, IFN-γ, IL-2, and IL-4, proinflammatory mediators, TNF-α, IL-1β and IL-6, and the TH1 mediators IL-12p40 and IL-10. Lower limits of detection are between 15–32pg/mL.
3.7. RNA Isolation and Real-time RT-PCR
Lung and spleen isolated from mice at day 7 post-challenge were sectioned and stored at −20°C in 500µL of RNA-Bee™ (Tel-Test, Inc. Friendswood, TX). RNA was isolated, DNase treated (Invitrogen, Carlsbad, CA), and 1µL RNA was used for transcription to cDNA [38]. Real time (Quantitative) PCR (RT-PCR) was performed utilizing the 7700 Sequence Detector (Applied Biosystems, Foster City, CA) [39–41]. Specific quantitative assays for β-actin, IFN-γ and TNF-α followed the recommended guidelines based on sequences from Genbank using reported sequences [37]. The limit of sensitivity for all assays was between 190 and 200 molecules, with approximately 95% assay efficiency. Data were normalized to β-actin and reported as fold change compared against non-immunized controls.
3.8. Histological techniques
The histological analysis of the lung was performed on day 28 and day 65 following aerosol infection. Lungs were fixed in 10% formalin and embedded in paraffin using standard techniques. Sections, 5µm thick, were stained with hematoxilin and eosin (H&E) and subsequently reviewed histologically; the pathologist viewing and interpreting the slides was blind to the type of experiment and treatment. Lungs from at least 6 mice of each group were analyzed. On day 65, histologic analysis of occlusion was performed in a blinded manner; 2–3 sections per block from 6–10 mice per time point for each group were digitized and analyzed using digital software (NIH Image, Prism-GraphPad). Relative area of occluding lesions was obtained, and calculated as a percent of total tissue area representented for each section.
3.9. Statistics
All vaccination protocols were repeated 2 or more times. All assaying experiments were repeated in triplicate (eg. ELISA determinations were performed using triplicate wells to calculate average ± standard deviation. Statistical analyses were carried out using OneWay ANOVA, and significance was reported at p<0.05, <0.01, and <0.001. Comparisons were made between the BCG/lactoferrin immunized groups, and mice either immunized with BCG alone or non-immunized controls.
4. Results
Lactoferrin was examined as an adjuvant to augment the BCG vaccine. The immunization schedules compared different vaccine protocols of the BCG alone and BCG admixed with lactoferrin. Protocols varied in formulation of vaccine (emulsion vs. saline), in dosing (single vaccination vs. prime-boost), and in time of challenge post final vaccination (2 or 4 weeks). A summary of vaccination protocols is provided in Table I.
Table I.
Overview of vaccination protocols
| Vaccine Protocol | Formulation | Times Immunized | Time of challenge post vaccination |
|---|---|---|---|
| A | emulsion | one time | 2 weeks |
| B | saline | one time | 2 weeks |
| C | saline | one time | 4 weeks |
| D | saline | prime & 2 weeks boost | 4 weeks |
4.1. BCG vaccination with lactoferrin increases protection against challenge with virulent M. tuberculosis, in both emulsion (Protocol A) and saline (Protocol B) vaccine formulations
The adjuvant activity of lactoferrin to affect the BCG vaccine was first compared when administered in an emulsion (IFA), or when administered in a saline vehicle. One group of mice was immunized with BCG emulsified in IFA with or without lactoferrin (Protocol A). A second set of mice was immunized with BCG in saline, with or without lactoferrin (Protocol B). To examine the effects of immunization on host protection against MTB infection, mice were then aerosol challenged with Erdman MTB (<100 CFU) at 2 weeks post-immunization. Mice were sacrificed at 4 weeks post-challenge, and organs isolated for CFU and histopathologic analysis. Comparisons were made to non-immunized mice aerosol challenged at the same time.
Organ bacterial load
It was previously demonstrated that emulsifying lactoferrin with BCG in IFA enhanced host protective responses against subsequent challenge with MTB, as observed by decreased organ bacterial loads and increased IFN-γ production during splenic recall to BCG antigens [25]. In that protocol, bacterial load was assessed in the lung, the primary site of infection, and the spleen, a representative site of organism dissemination, at 28 days post aerosol challenge (Fig. 1) in emulsion vehicle immunized mice. C57B1/6 mice immunized with BCG/IFA (4.48 +/− 0.26 Log CFU/organ) or BCG/lactoferrin/IFA (4.5 +/− 0.27 Log CFU/organ) demonstrated decreased lung CFU by greater than one-half Log compared to the non-immunized group (Log 5.12 +/− 0.26) (Fig. 1A). Examination of splenic bacterial load revealed that mice immunized with BCG/IFA/lactoferrin lowered organ CFU (2.84 +/− 0.27 Log CFU/organ) when compared to mice immunized with BCG/IFA (3.39 +/− 0.5 Log CFU/organ) (p=0.075). Significant reduction in CFU was evident (p<0.05) compared to the non-immunized group (4.63 +/− 0.42 Log CFU/organ) (Fig. 1B).
Fig. 1. Lung and splenic bacterial load post-challenge with MTB in mice immunized with BCG and lactoferrin adjuvant: Comparison of IFA and saline formulations.
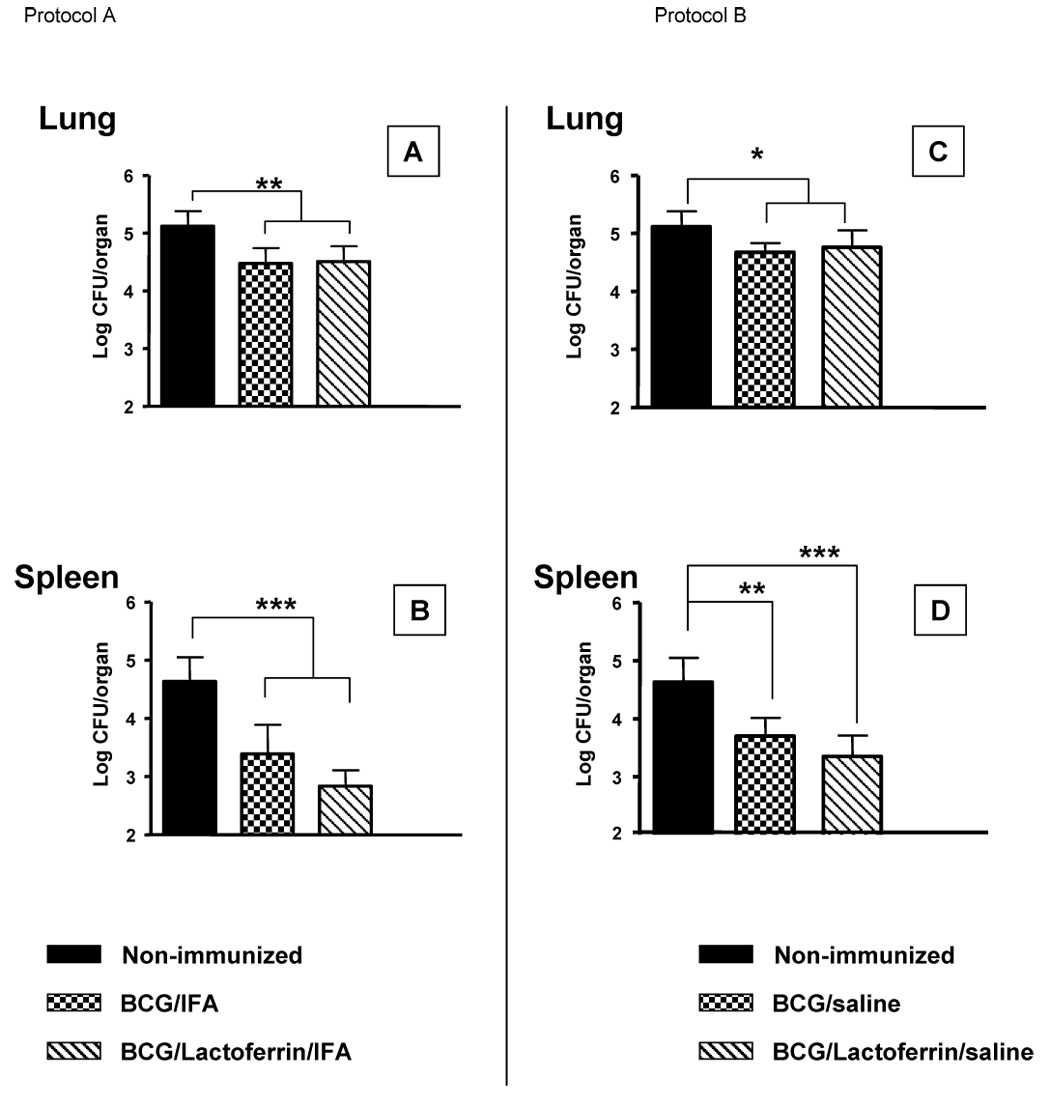
C57BL/6 were immunized once with BCG (1×106 CFU/mouse), or BCG and lactoferrin (100 µg/mouse). At 2 weeks post-immunization, mice were aerosol challenged with Erdman MTB (<100 CFU/mouse). Mice were sacrificed at day 28 post-challenge; lung and spleen were assessed for organ bacterial load. The left panel (A,B) depicts results when the vaccine was formulated in Incomplete Freund’s Adjuvant; the right panel (C,D) shows results of mice immunized with vaccine in a saline formulation. Comparisons were also made to non-immunized control infected mice. At least 6 mice were infected for each group. Average CFU per organ ± standard deviation shown; *p<0.05, **p<0.01, ***p<0.001; indicated comparisons made to BCG-immunized or non-immunized infected mice.
While IFA does not possess many of the side effects of CFA, which includes abscess development at the injection site, local tissue necrosis, and pain [42], it remains undesirable for human use. To further develop lactoferrin as an adjuvant to enhance the efficacy of the BCG vaccine, the adjuvant activity of lactoferrin was next examined when admixed with BCG in a saline formulation in the absence of emulsifier (Freund’s adjuvant). C57BL/6 mice were left non-immunized or immunized, once, with 100 µL of BCG (1×106 CFU/mouse), or BCG/lactoferrin (100 µg) in saline. Mice were infected via aerosol with Erdman MTB at 2 weeks post-immunization. At day 28 post-challenge, mice were sacrificed and lungs and spleens collected to assess for bacterial growth. Lung CFU in mice immunized with BCG (4.68 +/− 0.16 CFU/organ) and BCG/lactoferrin (4.76 +/− 0.29 Log CFU/organ) were decreased compared to the non-immunized group (5.12 +/− 0.26 Log CFU/organ) (Fig. 1C). Mice immunized with BCG (3.7 +/− 0.31 Log CFU/organ) and BCG/lactoferrin (3.35 +/− 0.36) also showed a decrease in splenic CFU compared to the non-immunized (4.63 +/− 0.42 Log CFU/organ) (Fig. 1D). Mice immunized with lactoferrin alone (without BCG) in saline demonstrated no differences from non-immunized mice in lung or splenic CFU (data not shown).
Lung histopathology
Reduced pulmonary histopathology was evident in lactoferrin immunized mice following challenge with MTB. Histological examination at day 28 post-challenge of lungs from non-immunized mice showed an inflammatory granulomatous response to MTB infection, displaying large clusters of lymphocytes and macrophages (Fig. 2A). Mice immunized with BCG/IFA showed granulomas that appeared slightly smaller in size compared to the non-immunized group (Fig. 2B). The smaller sized granulomas were also observed in lungs from mice immunized with BCG/lactoferrin/IFA, with lymphocytic clusters that appeared more tightly focused (Fig. 2C). In addition, mice that received BCG with lactoferrin had less inflammation and proteinaceous fluid in alveoli spaces.
Fig. 2. Lung histopathology in mice immunized with BCG and lactoferrin.

Mice immunized as in Figure 1 legend demonstrated reduced histological manifestation of disease in the BCG and lactoferrin immunized groups. Pathology from the unvaccinated control mice (A) is compared with different vaccinated groups. The left panel depicts histopathology from IFA formulated vaccinated mice (B,C); the right panel depicts saline formulation of vaccine (D,E). While BCG formulated in IFA demonstrated reduction in granulomatous response over the saline formulated counterpart, both demonstrated more inflammation than seen in the lactoferrin adjuvant immunized groups (C, E). Marked reduction in granulomatous response was evident in both of the lactoferrin immunized groups. Tissue was embedded, sectioned and stained with H&E (40x). Representative histology from 6 mice per group.
Similarly, in the saline experiments, MTB infection of non-immunized mice resulted in development of granulomas comprised of loosely associated clusters of lymphocytes and macrophages. The BCG immunized group formed granulomas that generally appeared to be smaller, compared to the non-immunized group, with a visible increase in lymphocytic infiltration (Fig. 2D). Vaccination with BCG in the presence of lactoferrin developed granulomas that appeared to be comprised with a higher lymphocytic content compared the non-immunized mice (Fig. 2E). In addition, MTB infected mice immunized with BCG/lactoferrin exhibited a markedly reduced inflammatory pathology in the lungs with granulomas surrounded by normal, non-inflamed, parenchyma. While immunization with the saline formulation of BCG/lactoferrin did not decrease organ bacterial load, an improved lung histopathology was observed compared to the BCG immunized group.
Splenic recall to BCG antigens
Immune responses generated to mycobacterial antigens by immunization were examined by splenic recall against BCG. Two weeks post-immunization, splenocytes were isolated and stimulated with BCG antigens in vitro. Supernatants were collected and analyzed by ELISA for T-cell and monocyte derived cytokines.
Splenocytes from mice immunized with lactoferrin added to BCG/IFA produced higher IFN-γ levels (888 +/− 94 pg/mL) during recall with BCG antigen when compared to splenocytes from both the BCG/IFA immunized (165 +/− 175 pg/mL) and non-immunized (47 +/− 23 pg/mL) immunized mice (Fig. 3, A–D). All immunized groups produced IL-2 in response to BCG with similar responses for all groups. IL-4 production was not detected (data not shown). Production of proinflammatory mediators was elevated from splenocytes isolated from mice immunized with BCG/lactoferrin/IFA. Production of TNF-α was significantly (p<0.05) increased during recall with splenocytes isolated from mice immunized with BCG/lactoferrin/IFA (1082 +/− 527 pg/mL) compared to the non-immunized group (409 +/− 140 pg/mL). Significantly higher levels of IL-6 (p<0.05) were also observed in splenocytes from immunization with BCG/lactoferrin/IFA (540 +/− 294 pg/mL) compared to BCG alone immunized controls (166 +/− 294 pg/mL). Althoug low, the production of IL-1β was also significantly different (p<0.05) between the BCG/lactoferrin/IFA (37 +/− 8 pg/mL) and the BCG/ IFA (11 +/− 4 pg/mL) groups.
Fig. 3. Cytokine and proinflammatory recall response to BCG antigens in BCG immunized mice with lactoferrin adjuvant.
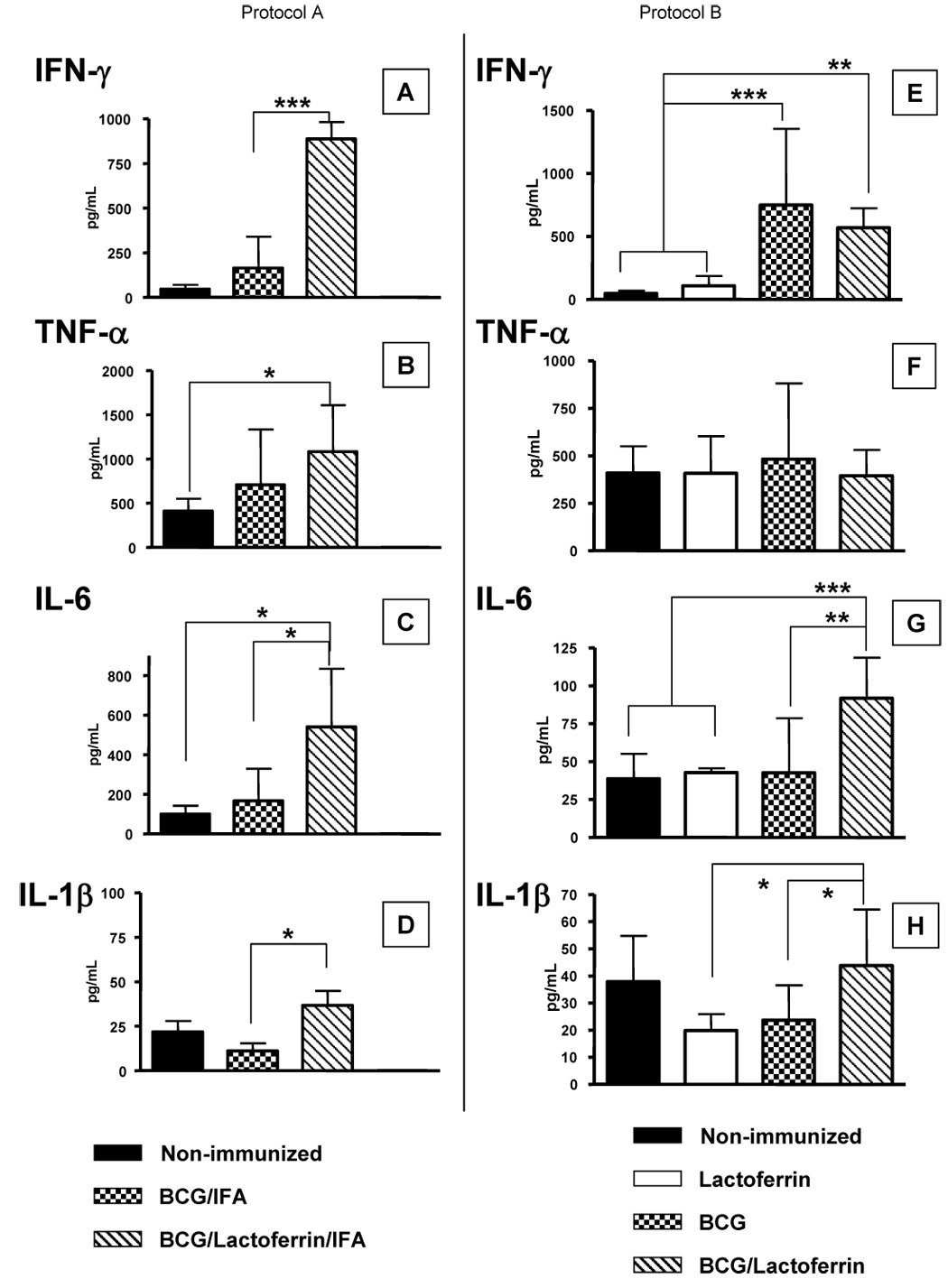
IFN-γ, TNF-α, IL-6, and IL-1β recall responses to BCG antigens were assessed from mice immunized with BCG or BCG/lactoferrin. Results shown indicate splenic responses from mice 2 weeks post single administration of vaccine formulated in IFA (Protocol A, A–D) or formulated in saline (Protocol B, E–H); comparisons in each set are made to non-immunized control mice. Cytokines shown as average ± standard deviation for at least 4 mice per group, done in triplicate; *p<0.05, **p<0.01, ***p<0.001; indicated comparisons made to BCG-immunized or non-immunized infected mice.
Recall responses were also performed on splenocytes isolated from the non-emulsion (saline) immunized mice given BCG or given BCG/lactoferrin, at 2 weeks post-immunization, and comparisons were made to non-immunized controls. Splenocytes were stimulated with BCG antigen and supernatants were analyzed. Significantly higher production of IFN-γ was demonstrated in response to antigen, from splenocytes isolated from mice immunized with BCG (748 +/− 647 pg/mL) or BCG/lactoferrin (570 +/− 154 pg/mL), compared to the non-immunized groups (Fig. 3E). There was an increase in IL-2 from antigen-stimulated splenocytes isolated from the BCG/lactoferrin immunized group (25 +/− 8 pg/mL) compared to the non-immunized (12 +/− 3 pg/mL), and BCG (13 +/− 4 pg/mL) immunized groups. However, overall production of IL-2 was low. No IL-4 was detected (data not shown).
TNF-α, IL-6 and IL-1β responses were also monitored in the saline vaccinated groups (Fig. 3, F–H). TNF-α production in response to BCG antigen stimulation was not significantly different from splenocytes isolated from all groups of mice examined. Splenocytes isolated from mice immunized with BCG/lactoferrin increased IL-6 production (92 +/− 27 pg/mL) upon antigen stimulation when compared to splenocytes from mice immunized with only BCG (43 +/− 36 pg/mL); of interest was that immunization in this manner (saline alone without IFA) led to overall less IL-6 produced. BCG antigen stimulated splenocytes from non-immunized mice (38 +/− 17 pg/mL) had similar level of IL-1β compared to mice immunized with BCG/lactoferrin (44 +/− 21 pg/mL); production of IL-1β was significantly decreased in splenocytes from the BCG immunized group (24 +/− 13 pg/mL) compared to the BCG/lactoferrin group.
4.2. BCG vaccination with lactoferrin increases protection against challenge with virulent M. tuberculosis, in saline vaccine formulation given once (Protocol C) or in prime-boost format (Protocol D)
TB vaccines are useful when given as a one time injection or when administered using various schedules of prime-boost protocols [10, 14, 15, 43–49]. The next experiments examined the BCG/lactoferrin saline immunization protocol to generate protection against MTB infection when mice were immunized once (Protocol C), or when boosted prior to infection (Protocol D). Mice were immunized with BCG (1×107 CFU/mouse) or BCG/lactoferrin (100µg/mL). Control mice were not immunized. All mice were challenged with virulent Erdman MTB at 4 weeks post-immunization, at a time when non-specific responses due to immunization activation would be limited.
Organ bacterial load
Lung CFU were examined first in mice that were immunized once, then challenged with virulent MTB, strain Erdman (Fig. 4A). Organism load was reduced in both the BCG immunized mice (4.9 +/− 0.2 Log CFU/organ) and BCG/lactoferrin immunized mice (4.95 +/− 0.26 Log CFU/organ), compared to the non-immunized group (5.54 +/− 0.27 Log CFU/organ) at day 28 post-challenge. There were no significant differences in lung CFU from MTB challenged mice at day 65 post-challenge in all groups examined. Splenic CFU changes over time post-challenge followed a similar pattern (Fig. 4B). Mice immunized with BCG (3.33 +/− 0.42 Log CFU/organ) and BCG/lactoferrin (3.32 +/− 0.29 Log CFU/organ) demonstrated reduced splenic bacterial load compared to the non-immunized group (4.49 +/− 0.55 Log CFU/organ) at day 28 post-challenge. The differences in splenic CFU were resolved by day 65 post-challenge relative to the non-immunized controls.
Fig. 4. Bacterial load post-challenge with MTB in mice immunized with BCG and lactoferrin adjuvant: Comparison of single vaccination vs. boosting.

C57BL/6 were immunized once with BCG, or with BCG and lactoferrin, formulated in saline (Protocol C, left panel), and subsequently aerosol challenged with Erdman MTB 4 weeks later. Alternatively, mice were boosted at 2 weeks post-primary immunization, and then challenged 4 weeks later (Protocol D, right panel). Mice were sacrificed at days 7, 28, and 65 post-challenge, and lung (A,C) and spleen (B,D) tissue were assessed for organ bacterial load. Comparisons were also made to non-immunized control infected mice. At least 6 mice were infected for each group. Average CFU per organ ± standard deviation shown; *p<0.05, **p<0.01, ***p<0.001 compared to BCG immunized or non-immunized control infected mice at indicated times.
In mice immunized and boosted prior to challenge, greater differences were seen between BCG alone and the BCG/lactoferrin groups (Fig. 4C,D). At day 28 post-challenge, a significant (p<0.001) decrease in lung CFU was observed in mice immunized and boosted with BCG (5.03 +/− 0.22 Log CFU/organ) and BCG/lactoferrin (4.88 +/− 0.16 Log CFU/organ) when compared with the non-immunized (5.54 +/− 0.27 Log CFU/organ) group. At day 65 post-challenge, mice immunized and boosted with BCG (5.54 +/− 0.34 Log CFU/organ) or BCG/lactoferrin (5.19 +/− 0.29 Log CFU/organ) significantly (p<0.05) decreased lung bacterial load compared to the non-immunized group (6.13 +/− 0.77 Log CFU/organ). In addition, the decrease in lung CFU from mice immunized and boosted with BCG/lactoferrin was significant (p<0.05) compared to the BCG immunized and boosted group. Examination of splenic CFU at day 28 post-challenge showed mice immunized and boosted with BCG (3.9 +/− 0.51 Log CFU/organ) or with BCG/lactoferrin (3.00 +/− 0.49 Log CFU/organ) decreased splenic CFU compared to the non-immunized group (4.49 +/− 0.55 Log CFU/organ). Mice immunized and boosted with BCG/lactoferrin had a further 1 Log decrease in splenic CFU compared to mice immunized and boosted with BCG only.
Lung histopathology
Examination of lung pathology at day 65 post-challenge showed that non-immunized mice developed granulomas comprised of clusters of lymphocytes and activated foamy macrophages (Fig. 5A). Non-immunized controls demonstrated granulomas surrounded by an overall inflamed parenchyma characterized by the thickened alveoli walls. Lungs from MTB infected mice immunized only with BCG demonstrated inflammatory responses that differed from the non-immunized group, showing granulomatous response with increased lymphocytic content. There was no obvious difference in histopathology in lungs from mice infected after single immunization (Fig. 5B), or infected after a second administration of vaccine (Fig. 5E). In contrast, lungs from MTB infected mice immunized with BCG/lactoferrin developed granulomas that appeared to be smaller in size compared to both the non-immunized and BCG alone immunized groups (Fig. 5C,D). While the granulomatous response from mice immunized with BCG/lactoferrin after MTB infection was similarly comprised of lymphocytic and foamy macrophage clusters, the surrounding parenchyma appeared to be considerably less inflamed with normal alveoli wall architecture. Overall, the MTB challenge of BCG/lactoferrin immunized mice demonstrated reduced lung inflammation.
Fig. 5. Mice immunized with BCG and lactoferrin demonstrate diminished inflammation and destructive pulmonary histopathology upon challenge with virulent MTB.
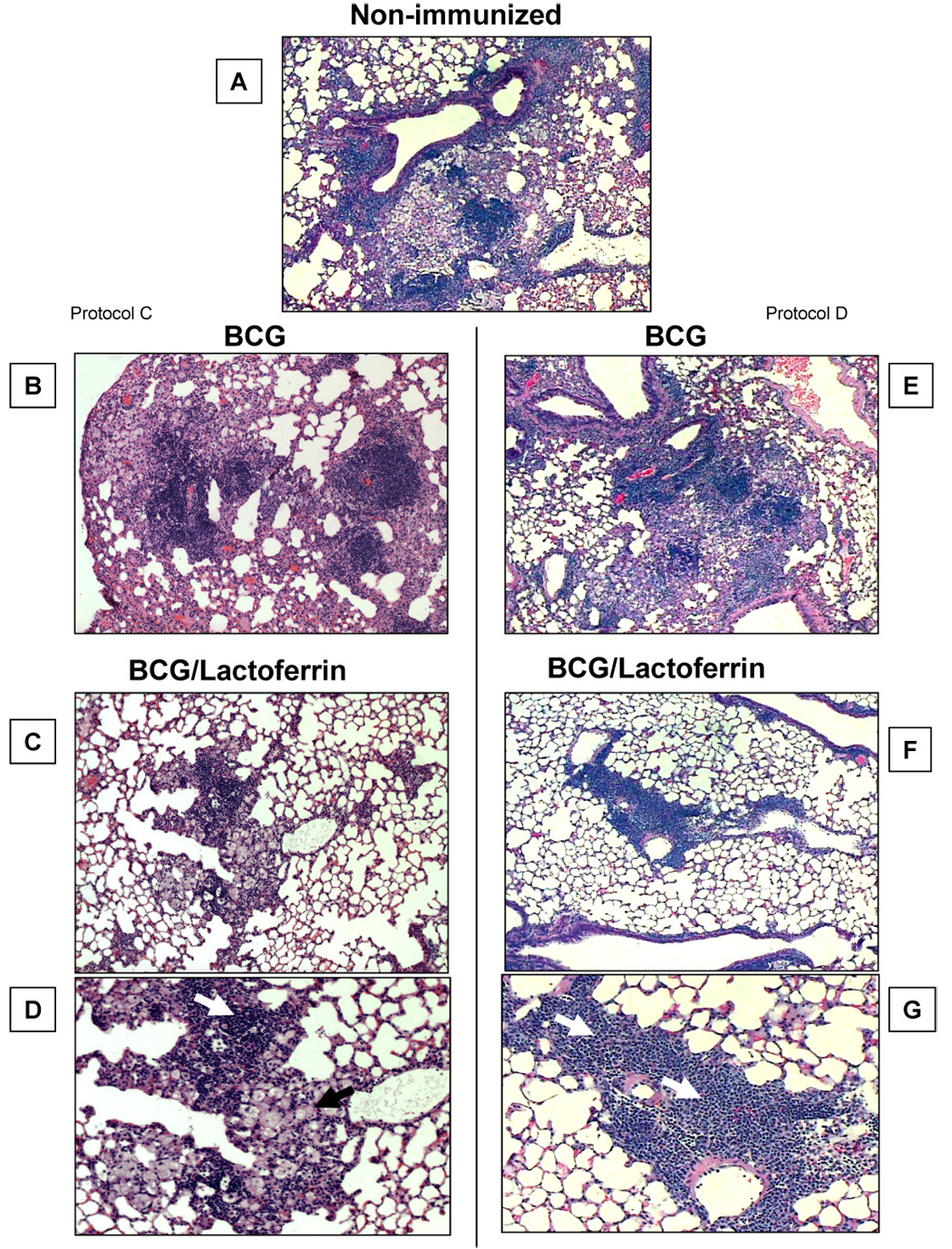
C57BL/6 mice immunized as in Figure 4 demonstrated reduced histological manifestation of disease in the BCG/lactoferrin immunized groups at day 65 post challenge. The top panel (A) depicts histopathology from a non-immunized control, and is compared to mice singly immunized with vaccine formulated in saline (left panel, B–D), or compared to tissue from mice vaccinated twice (right panel, E–G). Boosting with BCG alone led to a small but detectable reduction in granulomatous response over the saline formulated counterpart, however both demonstrated more inflammation than seen in the lactoferrin adjuvant immunized groups. The lactoferrin adjuvant immunized groups revealed a striking reduction in granulomas with evidence of lymphocytic clusters (white arrowhead) and contained focal pockets of inflamed monocytes (black arrowhead). Tissue was embedded, sectioned and stained with H&E (40x for A–C, E–F; 100x for D,G). Representative histology from 6 mice per group.
The immunized and boosted groups (Protocol D) demonstrated slightly better histopathology than the single lactoferrin immunized group (Protocol C). Upon aerosol infection with Erdman MTB, lungs from singly BCG/lactoferrin-immunized mice, at day 65 post-challenge, had an apparent reduction in lung inflammatory responses, showing normal lung parenchyma surrounding granulomas that were visually highly lymphocytic in composition (Fig. 5F,G). There were foamy macrophages present within the developed granulomas from the BCG/lactoferin group, however the foamy macrophage clusters appeared smaller and more compact compared to the non-immunized and BCG immunized mice.
The apparent reduction in inflammatory pathology was semi-quantitated by analysis of lung weight index (LWI), and quantitatively by measuring the area of the lung occluded by granulomas and lesions developed during the infective process. Mice immunized and boosted with BCG in the presence of lactoferrin had significantly and consistently lower LWI (p<0.001), at days 28 and 65 compared to mice immunized with BCG or the non-immunized group (Fig. 6A). An analysis was undertaken to quantitate changes in pathological manifestation due to infection. The BCG/lactoferrin immunized mice demonstrated significant reduction (p<0.05) in lung occlusion at 65 days post infection with the virulent organisms (Fig. 6B), compared to BCG alone vaccinated or the non-immunized controls.
Fig. 6. Quantitative analysis of inflammation in immunized mice.

Lung weight index (LWI) was calculated at day 7, 28 and 65 post challenge of mice immunized with BCG or BCG/lactoferrin, and compared to non-immunized challenged mice (A). On day 65, histological sections were assessed for changes in lung pathology by quantitative evaluation of inflammatory lesions, represented as percent occlusion of tissue (B). Results are shown as average ± standard deviation for at least 6 mice per group; *p<0.05, **p<0.01; indicated comparisons made to BCG-immunized infected mice.
Splenic recall
BCG antigen specific splenic immune responses generated by immunization with BCG or BCG/lactoferrin were compared. At 6 weeks post-immunization, splenocytes were isolated and stimulated with BCG antigen; T cell and monocytic cytokines were subsequently analyzed by ELISA. Mice that received BCG/lactoferrin vaccination increased production of IFN-γ (477 +/− 329 pg/mL) in response to antigen compared to the BCG immunized (182 +/− 140 pg/mL) or non-immunized (16 +/− 4 pg/mL) groups (Fig 7A). IL-12p40 was moderately augmented in splenocytes isolated from mice immunized with BCG (42 +/− 9 pg/mL) and BCG/lactoferrin (40 +/− 3 pg/mL) compared to the non-immunized group (34 +/− 4 pg/mL) (Fig 7B). Production of IL-4 was decreased from splenocytes isolated from BCG and BCG/lactoferrin immunized groups (Fig. 7C).
Fig. 7. Cytokine production to BCG antigens in BCG/lactoferrin immunized mice.

IFN-γ, IL-12 and IL-4 recall responses to BCG antigens were assessed from mice immunized with BCG or BCG/lactoferrin. Results shown indicate splenic responses from mice at 4 weeks post single administration of vaccine formulated in saline given once (Protocol C, A–C)) or from mice that were immunized and boosted (Protocol D, D–F)); comparisons in each set are made to non-immunized control mice. Cytokines shown as average ± standard deviation for 4–6 mice per group, done in triplicate; *p<0.05, **p<0.01, ***p<0.001; indicated comparisons made to BCG-immunized or non-immunized infected mice.
IFN-γ production was increased after stimulation with BCG antigen from splenocytes isolated from mice immunized and boosted with BCG/lactoferrin (778 +/− 178 pg/mL) compared to the BCG immunized (430 +/− 613 pg/mL) and non-immunized (16 +/− 4 pg/mL) groups (Fig. 7D). IL-12p40 production was moderately enhanced in antigen-stimulated splenocytes isolated from mice immunized and boosted with BCG/lactoferrin (45 +/− 4 pg/mL) compared to splenocytes isolated from mice immunized and boosted with BCG (35 +/− 7 pg/mL) and the non-immunized group (34 +/− 4 pg/mL) (Fig. 7E). IL-4 was decreased in both BCG (7 +/− 3 pg/mL) and BCG/lactoferrin (7 +/− 4 pg/mL) immunized groups compared to the non-immunized (42 +/− 43 pg/mL) mice (Fig. 7F).
Stimulation with BCG antigen increased TNF-α production from splenocytes isolated from the BCG (420 +/− 88 pg/mL) and BCG/lactoferrin (369 +/− 54 pg/mL) immunized groups. This increase was significant (p<0.001) compared to the non-immunized group (211 +/− 54 pg/mL). Production of IL-6 was also increased from antigen-stimulated splenocytes isolated from mice immunized with BCG (181 +/− 73 pg/mL) and BCG/lactoferrin (172 +/− 30 pg/mL) compared to splenocytes isolated from non-immunized mice (73 +/− 22 pg/mL). No difference in production of IL-1β was observed in all groups analyzed. Production of IL-10 was increased only in the BCG immunized group (42 +/− 11 pg/mL). Splenocytes from the non-immunized (26 +/− 2 pg/mL) and BCG/lactoferrin (34 +/− 5 pg/mL) immunized mice had levels of IL-10 that was lower compared to the BCG immunized group.
Splenocytes from mice immunized and boosted with BCG/lactoferrin elevated production of TNF-α (596 +/− 57 pg/mL) after stimulation with antigen compared to mice immunized and boosted with BCG without lactoferrin (234 +/− 117 pg/mL) and the non-immunized group (301 +/− 59 pg/mL). Similarly, splenocytes from mice immunized and boosted with BCG/lactoferrin increased production of IL-6 (208 +/− 18 pg/mL) compared to non-immunized mice (73 +/− 22 pg/mL) and mice immunized and boosted with BCG without lactoferrin (140 +/− 112 pg/mL). There were no differences observed in IL-1β in all groups examined. Production of IL-10 was slightly increased in splenocytes from mice immunized and boosted with BCG (34 +/− 9 pg/mL) and BCG/lactoferrin (32 +/− 2 pg/mL) compared to the non-immunized group (26 +/− 2 pg/mL).
Organ mRNA level
To address if immunization using lactoferrin adjuvant would generate a rapid specific immune response following infection, lungs and spleens of immunized and boosted mice were assessed for levels of IFN-γ and TNF-α mRNA present at 7 days post infection with virulent organisms. Comparison of the BCG/lactoferrin immunized group to the BCG-alone immunized groups or to the non-immunized grooup revealed significant increases in IFN-γ mRNA. Specifically, IFN-γ mRNA in the lung was elevated 13.2-fold (+/− 3.7) compared to the non-immunized control mice, which was significantly different (p<0.05) from the BCG-alone immunized group (6.8-fold +/− 3.6) (Fig. 8A). Similar effects were demonstrated in the spleen (Fig. 8B). Analysis of TNF-α mRNA levels in both tissues also revealed relative increases at 7 days post challenge in both lung and spleen (5.2-fold +/− 3.4; 1.51-fold +/− 0.5 respectively), however results were not significantly different to the BCG vaccinated or non-immunized control animals.
Fig. 8. IFN-γ and TNF-α mRNA at 7 days post infection in tissue of BCG/lactoferrin immunized mice.

Real time RT-PCR analysis of IFN-γ and TNF-α mRNA from C57BL/6 mice immunized and boosted with BCG alone or with BCG/lactoferrin were evaluated in lung tissue (top) or in spleen (bottom) at 7 days post infection with virulent MTB. Message levels are indicated as average ± standard deviation for at least 4 mice per group, shown as fold change vs. non-immunized controls; *p<0.05, **p<0.01, ***p<0.001; indicated comparisons made to BCG-immunized or non-immunized infected mice.
5. Discussion
The studies described here indicate that lactoferrin may be useful as an adjuvant to augment the BCG vaccine to protect against subsequent infection with virulent Mycobacterium tuberculosis. Furthermore, efficacy was demonstrated when lactoferrin was incorporated into a saline-based formulation, with histological protection when administered either as a single vaccination or when given in a prime-boost protocol. The main effect of lactoferrin to enhance the BCG vaccine is observed in the reduction of immune-related pathological tissue destruction in subsequently challenged mice.
There is intensive research into development of novel TB vaccines with increased efficacy relative to BCG. It can be argued for safety reasons that it would be beneficial to use the existing BCG vaccine if an adjunct component could be incorporated into the formulation which would drive protective responses and reduce infection induced pathology [14, 15, 50]. A common goal in improving the current vaccine is to increase generation of antigen-specific cell mediated immunity [34, 51]. Lactoferrin, an iron binding protein, is an excellent candidate for this process, and has demonstrated ability to promote T-cell mediated delayed-type hypersensitivity against a variety of antigens, including BCG [22, 23]. These experiments outlined here confirm this observation, and show lactoferrin capable of significantly enhancing the efficacy of the BCG vaccine and generating host protection against MTB infection, which culminated in reduction of destructive histopathology following infectious challenge.
The first set of protocols examined the adjuvant activity of lactoferrin to enhance the efficacy of BCG in an emulsion formulation (incomplete Freund’s adjuvant), compared to use of BCG in a saline formulation. The data described confirm that vaccination with BCG alone is more effective when administered in emulsion formulation compared to a saline-based preparation. Novel to these experiments, greater protective responses were seen when lactoferrin was added to both formulations with reduced CFUs, reduced pulmonary granulomatous response, and increased IFN-γ and proinflammatory recall responses to BCG antigens. Especially unique to these experiments was that lactoferrin added to BCG in saline was effective at limiting damaging pulmonary pathology. The elimination of IFA has a direct advantage in limiting toxic properties of emulsion-based vaccines and increased potential for use in larger vertebrates.
Overall, the lactoferrin adjuvant groups demonstrated marked lymphocytic involvement and smaller focal monocytic response in lung tissue at the later stages of infection. This higher level of lymphocytic response may explain the observation of slower progression of organism dissemination to splenic tissue. At 2 months after infection the lymphocytic recall responses towards BCG antigens were significantly elevated in the lactoferrin adjuvant groups. The most dramatic effects were seen when lactoferrin was given with BCG using a prime-boost protocol. A strong DTH response was generated, allowing a rapid and elevated increase in IFN-γ mRNA apparent by 7 days post infection.
In all protocols, addition of lactoferrin to the BCG vaccine improved mycobacterial antigen specific T-cell helper type 1 (TH1) response, indicated by the increase in IFN-γ protein production to BCG antigens. This is known to be crucial for host protection against MTB infection [52–54]. Hwang, et al. demonstrated in vitro that addition of lactoferrin on activated macrophages leads to increase IL-12 production while at the same time reducing IL-10 [25, 33]. Together, this indicates that lactoferrin could enhance generation of mycobacterial antigen responses towards the TH1 phenotype. In addition to a more rapid upregulation in IFN-γ mRNA response, lactoferrin admixed to the BCG vaccine generated higher levels of TNF-α, which has the potential to synergize with IFN-γ to direct development of the protective granulomatous response during MTB infection [55–57]. Granulomas are the protective mechanism enabling containment of MTB infection and prolonging host survival [55, 58–60]. Lactoferrin also promoted production of IL-12p40 from splenocytes isolated from BCG/lactoferrin immunized and boosted mice, increasing immune response that supported the development of TH1 immunity during MTB infection [61–63]. Finally, the increase in IL-6 production from BCG antigen-stimulated splenocytes isolated from mice immunized and boosted with BCG/lactoferrin is puzzling, as IL-6 is typical of a TH2 response [64], and has inhibitory functions in macrophages relative to mycobacterial antigen presentation [65, 66]. In this instance, IL-6 may be responsible for inhibiting uncontrolled inflammatory responses during MTB infection [59].
Analysis of vaccine efficacy typically relies only on reduction of organism load in tissue following subsequent infection with virulent mycobacterium. Although tissue specific CFU reduction is a good indicator, the correlation with disease should also be evaluated by histopathology, as controlling pathological damage is key to surviving tuberculosis. Indeed, animals are able to survive with markedly elevated organism loads, provided that protective granulomatous responses occur, allowing sequestration of organisms and localization of inflammatory response [36, 59, 67]. In these experiments, control of MTB proliferation in the lungs correlated well with formulation of granulomas, with limited presence of complex and activated cellular clusters of macrophages and lymphocytes in the lactoferrin adjuvant groups. A fundamental flaw in the mouse model is the transient effect of CFU reduction. In these experiments, differences in overall CFU reduction occurred between protocols; only the boosted immunization lactoferrin adjuvant groups maintaining a significant advantage (albeit a small change) over the BCG alone vaccinated group. Even so, despite the re-emergence of organisms in the lung at the later time points, the reduction in immune mediated pulmonary tissue damage was striking at day 65 with clear positive advantages for the animal in associated lung function. The limited pathology was a robust and long-lived response, with reduced damage to lung tissue observed through at least 120 days (S-A Hwang, personal communication).
The findings here indicate that lactoferrin should be included as a useful tool in the developing vaccine arsenal to combat mycobacterial infection. Many novel vaccine candidates demonstrating superiority over the existing BCG vaccine are genetically modified live BCG recombinants [7, 9, 10, 15, 44, 47, 48], with a general realization of the requirement to maintain the multitude of cross-reactive mycobacterial antigens. In these studies, bovine lactoferrin was used to significantly enhance the current BCG vaccine. There is antigenic variation between human, bovine and murine lactoferrins (approximately 70–77% direct protein sequence match between all three molecules), which may impact responses in mice [68]. Future studies should examine responses using human or humanized lactoferrins. In conclusion, these results suggest that lactoferrin is a useful adjuvant to stimulate cell mediated immunity during BCG vaccination, and may form the basis to use lactoferrin as an adjuvant with the “next generation” of antituberculosis vaccines. Furthermore, lactoferrin admixed with BCG and administered as a single-shot vaccine, or used in a prime boost vaccine formulation, holds high potential as a vaccine candidate to improve response to mycobacterial immunogens. We therefore recommend further testing and analysis of lactoferrin for use with current and future MTB vaccines.
6. Acknowledgements
This work was accomplished in large part due to support from NIH grant R41AI51050-01 & R42-AI051050-02. We thank Michal Zimecki, Ph.D. (Department of Experimental Therapy, Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland) and David N. McMurray, Ph.D. (Department of Medical Microbiology and Immunology, Texas A&M University System Health Science Center) for helpful conversations, insights and data discussion, and critical evaluation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- 1.Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis. 1999;79(4):243–250. doi: 10.1054/tuld.1999.0206. [DOI] [PubMed] [Google Scholar]
- 2.Behr MA. BCG--different strains, different vaccines? Lancet Infect Dis. 2002;2(2):86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 3.Maher D, Raviglione M. Global epidemiology of tuberculosis. Clin Chest Med. 2005;26(2):167–182. doi: 10.1016/j.ccm.2005.02.009. v. [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96(1 Pt 1):29–35. [PubMed] [Google Scholar]
- 5.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2(3):200–207. [PubMed] [Google Scholar]
- 6.Brennan MJ. A New Generation of Tuberculosis Vaccines. In: Quadros CAd., editor. Preventing Disease and Protecting Health. Pan American Health Organization; 2004. pp. 177–182. [Google Scholar]
- 7.Doherty TM. Real world TB vaccines: clinical trials in TB-endemic regions. Vaccine. 2005;23(17–18):2109–2114. doi: 10.1016/j.vaccine.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 8.McMurray DN. A coordinated strategy for evaluating new vaccines for human and animal tuberculosis. Tuberculosis (Edinb) 2001;81(1–2):141–146. doi: 10.1054/tube.2000.0265. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26(12):660–667. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A. 2000;97(25):13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31 Suppl 3:S64–S67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 13.McMurray DN. Recent progress in the development and testing of vaccines against human tuberculosis. Int J Parasitol. 2003;33(5–6):547–554. doi: 10.1016/s0020-7519(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 14.Haile M, Schroder U, Hamasur B, Pawlowski A, Jaxmar T, Kallenius G, et al. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine. 2004;22(11–12):1498–1508. doi: 10.1016/j.vaccine.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Chambers MA, Wright DC, Brisker J, Williams A, Hatch G, Gavier-Widen D, et al. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome) protects guinea pigs from lethal tuberculosis. Vaccine. 2004;22(8):1063–1071. doi: 10.1016/j.vaccine.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Baggiolini M, De Duve C, Masson PL, Heremans JF. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem Mol Biol Int. 1997;43(1):79–87. doi: 10.1080/15216549700203841. [DOI] [PubMed] [Google Scholar]
- 18.Brock JH. The physiology of lactoferrin. Biochem Cell Biol. 2002;80(1):1–6. doi: 10.1139/o01-212. [DOI] [PubMed] [Google Scholar]
- 19.Brock J. Lactoferrin: a multifunctional immunoregulatory protein? Immunol Today. 1995;16(9):417–419. doi: 10.1016/0167-5699(95)80016-6. [DOI] [PubMed] [Google Scholar]
- 20.Iyer S, Lonnerdal B. Lactoferrin, lactoferrin receptors and iron metabolism. Eur J Clin Nutr. 1993;47(4):232–241. [PubMed] [Google Scholar]
- 21.Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. 1992;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74(3):183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 23.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2(4):475–486. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 24.Kruzel ML, Zimecki M. Lactoferrin and immunologic dissonance: clinical implications. Arch Immunol Ther Exp (Warsz) 2002;50(6):399–410. [PubMed] [Google Scholar]
- 25.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5(3):591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, et al. T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol. 1994;24(4):793–798. doi: 10.1002/eji.1830240403. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi H, Takakura N, Yamauchi K, Tamura Y. Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin Vaccine Immunol. 2006;13(2):239–245. doi: 10.1128/CVI.13.2.239-245.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakabayashi H, Kurokawa M, Shin K, Teraguchi S, Tamura Y, Shiraki K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci Biotechnol Biochem. 2004;68(3):537–544. doi: 10.1271/bbb.68.537. [DOI] [PubMed] [Google Scholar]
- 30.Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals. 2004;17(3):231–234. doi: 10.1023/b:biom.0000027697.83706.32. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84(3):423–432. [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186(1):39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang S-A, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin Modulation of IL-12 and IL-10 Response from Activated Murine Leukocytes. Jounal of Interferon and Cytokine Research. doi: 10.1007/s00430-007-0041-6. accepted 02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30 Suppl 3:S266–S270. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 35.Shibaki A, Katz SI. Induction of skewed Th1/Th2 T-cell differentiation via subcutaneous immunization with Freund's adjuvant. Exp Dermatol. 2002;11(2):126–134. doi: 10.1034/j.1600-0625.2002.110204.x. [DOI] [PubMed] [Google Scholar]
- 36.Actor JK, Breij E, Wetsel RA, Hoffmann H, Hunter RL, Jr, Jagannath C. A role for complement C5 in organism containment and granulomatous response during murine tuberculosis. Scand J Immunol. 2001;53(5):464–474. doi: 10.1046/j.1365-3083.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 37.Guidry TV, Olsen M, Kil KS, Hunter RL, Jr, Geng YJ, Actor JK. Failure of CD1D-/-mice to elicit hypersensitive granulomas to mycobacterial cord factor trehalose 6,6′-dimycolate. J Interferon Cytokine Res. 2004;24(6):362–371. doi: 10.1089/107999004323142222. [DOI] [PubMed] [Google Scholar]
- 38.Actor J. Quantitation of cytokine mRNA by flash-type biouminescence. In: O'Neill L, editor. Methods in Molecular Medicine: Interleukin Protocols. Totowa, NJ: Human Press; 2001. pp. 83–97. [Google Scholar]
- 39.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6(10):986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 40.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6(10):995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 41.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 42.Stills HF., Jr Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. Ilar J. 2005;46(3):280–293. doi: 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- 43.Ami Y, Izumi Y, Matsuo K, Someya K, Kanekiyo M, Horibata S, et al. Priming-boosting vaccination with recombinant Mycobacterium bovis bacillus Calmette-Guerin and a nonreplicating vaccinia virus recombinant leads to long-lasting and effective immunity. J Virol. 2005;79(20):12871–12879. doi: 10.1128/JVI.79.20.12871-12879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao L, Chen W, Zhang H, Wang X. Virulence, immunogenicity, and protective efficacy of two recombinant Mycobacterium bovis bacillus Calmette-Guerin strains expressing the antigen ESAT-6 from Mycobacterium tuberculosis. Infect Immun. 2003;71(4):1656–1661. doi: 10.1128/IAI.71.4.1656-1661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majlessi L, Simsova M, Jarvis Z, Brodin P, Rojas MJ, Bauche C, et al. An increase in antimycobacterial Th1-cell responses by prime-boost protocols of immunization does not enhance protection against tuberculosis. Infect Immun. 2006;74(4):2128–2137. doi: 10.1128/IAI.74.4.2128-2137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005;7(5–6):962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9(5):533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 48.Rao V, Dhar N, Tyagi AK. Modulation of host immune responses by overexpression of immunodominant antigens of Mycobacterium tuberculosis in bacille Calmette-Guerin. Scand J Immunol. 2003;58(4):449–461. doi: 10.1046/j.1365-3083.2003.01321.x. [DOI] [PubMed] [Google Scholar]
- 49.Wozniak TM, Ryan AA, Triccas JA, Britton WJ. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect Immun. 2006;74(1):557–565. doi: 10.1128/IAI.74.1.557-565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haile M, Hamasur B, Jaxmar T, Gavier-Widen D, Chambers MA, Sanchez B, et al. Nasal boost with adjuvanted heat-killed BCG or arabinomannan-protein conjugate improves primary BCG-induced protection in C57BL/6 mice. Tuberculosis (Edinb) 2005;85(1–2):107–114. doi: 10.1016/j.tube.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Srivastava I. Advances in vaccine adjuvants for infectious diseases. Curr HIV Res. 2003;1(3):309–320. doi: 10.2174/1570162033485195. [DOI] [PubMed] [Google Scholar]
- 52.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201(12):1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17(4):374–380. doi: 10.1016/j.coi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Logan KE, Chambers MA, Hewinson RG, Hogarth PJ. Frequency of IFN-gamma producing cells correlates with adjuvant enhancement of bacille Calmette-Guerin induced protection against Mycobacterium bovis. Vaccine. 2005;23(48–49):5526–5532. doi: 10.1016/j.vaccine.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162(6):3504–3511. [PubMed] [Google Scholar]
- 56.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 57.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 1997;27(12):3182–3190. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 58.Saunders BM, Cooper AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol Cell Biol. 2000;78(4):334–341. doi: 10.1046/j.1440-1711.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 59.Actor JK, Olsen M, Jagannath C, Hunter RL. Relationship of survival, organism containment, and granuloma formation in acute murine tuberculosis. J Interferon Cytokine Res. 1999;19(10):1183–1193. doi: 10.1089/107999099313136. [DOI] [PubMed] [Google Scholar]
- 60.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, et al. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol. 2001;167(12):6957–6966. doi: 10.4049/jimmunol.167.12.6957. [DOI] [PubMed] [Google Scholar]
- 62.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168(3):1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 63.Bastos KR, Alvarez JM, Marinho CR, Rizzo LV, Lima MR. Macrophages from IL-12p40-deficient mice have a bias toward the M2 activation profile. J Leukoc Biol. 2002;71(2):271–278. [PubMed] [Google Scholar]
- 64.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10(12):542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 65.Nagabhushanam V, Solache A, Ting LM, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J Immunol. 2003;171(9):4750–4757. doi: 10.4049/jimmunol.171.9.4750. [DOI] [PubMed] [Google Scholar]
- 66.VanHeyningen TK, Collins HL, Russell DG. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997;158(1):330–337. [PubMed] [Google Scholar]
- 67.Jagannath C, Hoffmann H, Sepulveda E, Actor JK, Wetsel RA, Hunter RL. Hypersusceptibility of A/J mice to tuberculosis is in part due to a deficiency of the fifth complement component (C5) Scand J Immunol. 2000;52(4):369–379. doi: 10.1046/j.1365-3083.2000.00770.x. [DOI] [PubMed] [Google Scholar]
- 68.Debbabi H, Dubarry M, Rautureau M, Tome D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J Dairy Res. 1998;65(2):283–293. doi: 10.1017/s0022029997002732. [DOI] [PubMed] [Google Scholar]


