Abstract
Sequential learning is an important aspect of cognitive processing. Neuropharmacological evidence acquired in laboratory animals suggests that striatal dopaminergic mechanisms may be important for processing of this form of learning. However, because experiments conducted on dopamine deficient patients have reported contradictory evidence, the role of dopamine and the striatum remains unclear in human sequential learning. We used a newly developed dynamic molecular imaging technique to determine whether striatal dopamine is released during performance of a sequential learning task. In this study we localized striatal regions where dopamine receptor ligand (11C-raclopride) was displaced from receptor sites, during performance of a motor sequence learning (serial reaction time) task. The results suggest that the task induces release of endogenous dopamine in the posterior two-third of dorsomedial aspect of left putamen and the anterior part of the body of caudate bilaterally. The activations of the left putamen and the right caudate coincided with the activations observed earlier during performance of a motor planning task. Since these activations are associated with the selection and execution of a response, the activation in the left caudate, which was not observed in motor planning, is probably associated with the detection of a change in the ‘context’, and in the formulation of a new ‘rule’. Thus, the results suggest that sequential learning involves two striatal dopaminergic mechanisms, one for the detection of a change in context, and the other for selection and execution of the response.
Sequential learning is involved in the processing of a variety of cognitive functions including linguistic expression, semantic sequencing, working memory, and procedural memory (Lashley, 1951; Tinaz et al., 2006). The evidence suggests that this form of learning involves dopaminergic neurotransmission. It has been shown that patients with altered dopamine neurotransmission perform poorly in tasks that involve learning of sequences (Jackson et al., 1995; Carbon et al., 2004), and animals lose the ability to learn sequential tasks following lesions of dopaminergic neurons (Aosaki et al., 1994a; Matsumoto et al., 1999). In addition, neuroimaging studies (fMRI and PET rCBF experiments) have found increased activation of the dopamine-rich striatum, during performance of sequential learning tasks (Hazeltine et al., 1997; Rauch et al., 1997; Peigneux et al., 2000; Daselaar et al., 2003). However, because neurons that are involved in dopamine-dependent mnemonic processing (tonically active neurons) comprise only 5% of the striatal neuronal population (Aosaki et al., 1994b; Aosaki et al., 1995), it is not known whether the activation observed in these experiments are due to dopaminergic or non-dopaminergic activity (for review see Packard and Knowlton, 2002). Indeed, a number of investigators have suggested that dopamine may not be involved in the processing of human sequential learning (Brooks, 1995; Honda et al., 1998; Van Der Graaf et al., 2004; Ashe et al., 2006). This suggestion is supported by the findings of a set of neuroimaging experiments that have not found any change in striatal activation during task performance (Brooks, 1995; Honda et al., 1998; Van Der Graaf et al., 2004; Ashe et al., 2006). Furthermore, at least one series of study-patients with focal lesions in the basal ganglia performed normally in a sequential learning task (Exner et al., 2002). Even though these observations are not in agreement with the findings of a majority of neuroimaging (Hazeltine et al., 1997; Rauch et al., 1997; Peigneux et al., 2000; Daselaar et al., 2003) and lesion (Jackson et al., 1995; Carbon et al., 2004) studies, these reports have led investigators to argue that the striatal activations observed in neuroimaging experiments were due to regional cerebral blood flow (rCBF) changes triggered by the activities of a complex ensemble of excitatory and inhibitory neurons in the striatum (e.g., Honda et al., 1998). Because of contradictory findings and opinions, the role of dopamine and the striatum in the processing of human sequential learning remains unclear. The lack of consensus has precluded formulation of an acceptable neural model of human sequential learning. There is therefore a need to investigate striatal and dopaminergic involvement using a method that is not dependent on rCBF changes, and that can provide relatively direct evidence of the involvement of dopamine and striatum in sequential learning.
This evidence can be acquired using a molecular imaging technique, which is widely used to characterize receptor population either in a disease state (Farde et al., 1988; Seeman et al., 1989) or after pharmacological intervention (Laruelle et al., 1995; Kapur et al., 2000; Volkow et al., 2001). In recent years, investigators have conducted experiments to examine the ability of molecular imaging techniques to detect neurotransmitters released following a behavioral or pharmacological challenge (Dewey et al., 1993; Koepp et al., 1998; Pappata et al., 2002; Martinez et al., 2003). In these experiments receptor binding of the ligand, measured during a control (resting) scan is compared with that measured during a second scan session performed after the challenge. Because the ligand is competitively displaced from receptor sites by endogenously released dopamine, a decrease in ligand binding in the second scan indicates that the challenge induced release of dopamine. However, the requirement of two separate scans, is not ideal for detection of changes during processing of a specific aspect of human cognition because the variation in baseline dopaminergic activity during the two scan sessions cannot be accounted for. Since two-scan approach significantly limits the sensitivity and reliability (Alpert et al., 2003; Badgaiyan et al., 2003b), changes in dopaminergic activity induced by a specific aspect of cognition can be detected reliably only if the changes are detected in a single scan session.
We have recently described a molecular imaging method for detection of task-induced changes in neurotransmission using data acquired in a single scan (Alpert et al., 2003; Badgaiyan et al., 2003b). Using this method, we have detected endogenously released dopamine during performance of a number of behavioral and cognitive tasks (Alpert et al., 2003; Badgaiyan et al., 2003a; Badgaiyan et al., 2003b; Badgaiyan et al., 2004; Badgaiyan et al., 2005; Badgaiyan et al., 2006a; Badgaiyan et al., 2006b; Fischman et al., 2006; Badgaiyan et al., 2007a; Badgaiyan et al., 2007b). Other investigators have also found that the method is sensitive and reliable (Christian et al., 2006). In these experiments, a tracer dose of a radioligand was administered intravenously, and the task was initiated after the ligand occupied dopamine receptors. Since dopamine competitively displaces the ligand from receptor sites, a decrease in the ligand concentration during task performance indicated task-induced release of endogenous dopamine. This method was used in the present study to examine dopamine transmission during processing of a sequential learning task. The study includes two experiments. The first experiment used a serial reaction time (SRT) task to elicit sequential learning (Nissen and Bullemer, 1987). The second experiment was a control (no activation) experiment, which was designed to examine possible biases in data acquisition and analyses techniques.
Materials and methods
The experiments were conducted on right-handed young healthy volunteers who had no history of a psychiatric or neurological disorder.
A. SRT Experiment
Eight right-handed volunteers of either sex (mean age 24.5 years; male 3) participated in this experiment. Handedness was tested using a modified Edinburgh handedness inventory (Oldfield, 1971). The task consisted of a control and a test condition. In the control, four boxes (2.5 cm × 2.5 cm) were presented on a computer monitor, and an asterisk appeared in one of the boxes randomly every 1250 msec, for 800 msec. Subjects were asked to press a key that corresponded to the location of marked box, as quickly and as accurately as possible. In the test condition stimuli were presented in a 12-element ambiguous sequence in which no component could be uniquely predicted by its predecessor sequence. Further, the sequence had the following constraints: equal frequency of each position, no direct repetitions, and no runs (e.g., 1234). The sequence used was 2-3-1-4-3-2-4-1-3-4-2-1. Stimulus exposure and frequency of presentation were the same in the control and test conditions. Each control and test block was administered for 2 min and 15 sec. A 15 sec rest was allowed after each block. There were 10 control and 4 test blocks in the experiment. To ensure that the task elicited implicit learning, we used a long sequence consisting of 12 elements. Further, a two-tier debriefing procedure was used to evaluate implicit nature of the task. First a Likert’s scale (Likert, 1932) was used to screen for awareness of the sequential nature of the stimuli. Thereafter, subjects showing awareness were asked to recall the sequence. If a subject failed to recall >25% of elements, the task was considered implicit. The control condition lasted for 25 min and the test was administered for 10 min immediately after the control. Volunteers were not aware of the switch between the control and test. Response time and the accuracy of response were recorded in each trial.
B. Control Experiment
In another group of young healthy volunteers of either sex (n=10; mean age=22.73; males=5), a control experiment was conducted to ensure that the data in the SRT task were not generated by a false positive bias in the data acquisition and/or analyses methods. In the control experiment, after intravenous injection of the ligand 11C-raclopride, volunteers were asked to lie on the scanner bed and stay still. They were not required to perform any task. The concentration of ligand was monitored dynamically using the acquisition protocol described in the SRT experiment. The demographics of volunteers, data analyses, and experimental procedures (except those related to the SRT paradigm) used in the control and SRT experiments were similar.
Scan protocol
After the volunteers were placed on the scanner bed in supine position, their heads were restrained using an inflatable pillow (Vaclok, CIVCO Medical Solutions, Orange City, IA). We used a neuroshield (Scanwell Systems, Montreal, Canada) to reduce detection of scattered photons from the body. A single intravenous bolus of 10–15 mCi (mean specific activity 983±129 mCi/μmole) of a specific dopamine receptor ligand 11C-raclopride was administered intravenously in the left anticubital vein over a period of 60 sec. The control condition of the task (in the SRT experiment), and the PET camera were started immediately after the injection. The PET data were acquired using an ECAT EXACT HR+ tomograph (operating in 3-D mode) in 30 sec frames during the first five min and in 60 sec frames thereafter.
Data Analyses
The PET images were reconstructed as 128×128×63 element volumes using a standard three-dimensional filtered back projection algorithm with corrections for photon attenuation, random coincidences, scatter, and dead time. To minimize residual effects of head movements, images were registered to align each frame to a common orientation, using the following procedure: First, all frames were smoothed with a 5 mm FWHM Gaussian filter, then the variation in spatiotemporal distribution was corrected by registration of temporally adjacent frames, and finally, using a transformation matrix all frames were aligned to a reference frame (the frame acquired at 25 min). A voxel-wise analysis of the data was carried out on each subject using a kinetic model (discussed below) designed to detect transient changes in ligand displacement. Using this model, quantitative maps of the kinetic parameters were generated for each volunteer. These data were then pooled to generate cohort mean. This involved elastic registration of the sum of the image data of each subject, to a standard template, using the statistical parametric mapping software (SPM99; Wellcome Department of Imaging Neuroscience, London). The transformation parameters were then applied to the parametric images to pool data across volunteers. A voxel-wise t-map was computed to localize voxels where increased rate of ligand displacement was observed after the task initiation (test condition). Finally, time-activity curves were drawn for the voxels showing maximum ligand displacement. The cerebellum was used as a reference region (because of paucity of dopamine receptors in this region), and a time activity curve for this region was drawn to estimate the clearance rate of free and nonspecifically bound ligand.
The Model
A detailed description of the modified kinetic model used to analyze PET data can be found in our earlier publication (Alpert et al., 2003). This model is basically a modified version of the simplified reference region model (SRRM), which accounts for time dependent changes in the ligand concentration (Friston et al., 1997). There was a need to modify the SRRM model because it assumes a steady physiological state throughout the experiment. This assumption is not compatible with the design of the current experiment, which includes two conditions: a control and a test. The modified model assumes that steady state was not maintained. To eliminate the assumption of steady state we allowed the dissociation rate of ligand to change in response to an altered synaptic level of neurotransmitter. This was done by introducing a term γ·exp(−τ(t−T))●ν(t−T) in the dissociation parameter of the SRRM. In the modified model, γ represents the amplitude of ligand displacement, τ accounts for initial burst release of dopamine, t denotes the measurement time, T is the time of change in transmitter level, and ν is the unit step function. Using the least squares fitting procedure on a voxel-by-voxel basis receptor binding parameters and γ are estimated. The null hypothesis assumes that the task did not elicit dopamine release and there was no change in the rate of ligand displacement (i.e., γ=0). This hypothesis was tested in each subject and the values for ‘γ’ were pooled across subjects to acquire a cohort mean and variance. The null hypothesis was rejected at the p=0.05 level. Additionally, we estimated the parameters that describe ligand transport and binding, and the time dependent effects elicited by the task. The solution of the differential equations describing the model for the instantaneous concentration history of the ligand has the following form:
where, CR is the concentration of radioligand in a region devoid of specific binding (reference), PET is the concentration of radioligand in a voxel with specific binding, R is the ratio of transport rates for the binding and reference regions, k2 describes the clearance of nonspecifically bound tracer from the voxel, and k2a includes information about dissociation from the receptor.
Results
The SRT Experiment
In this experiment response accuracy during the control (98.6%) and test (99.2%) conditions was high and statistically similar. The response time however, was significantly quicker (p<0.01) in the test (310 ± 26 msec) condition, in comparison with the control (405 ± 94 msec). Quicker response in the test suggested that the volunteers had learned the motor sequence (Figure 1). Further, implicit nature of the task was confirmed by debriefing questionnaires. Six of the eight volunteers were not aware that the stimuli were presented in a sequence in the test condition. Two volunteers who were aware of the sequential nature, failed to explicitly recall more than 25% consecutive elements. The task is generally considered implicit if the explicit recall is <50% of consecutive elements (Rauch et al., 1997).
Figure 1.
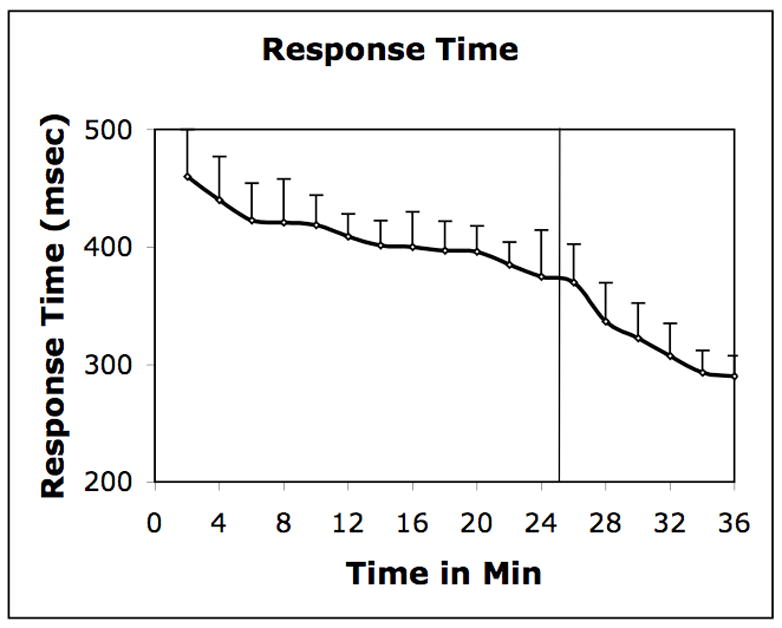
Response Time: Mean response time during the SRT task performance. Stimuli were presented randomly in the first 25 min and sequentially thereafter (vertical line).
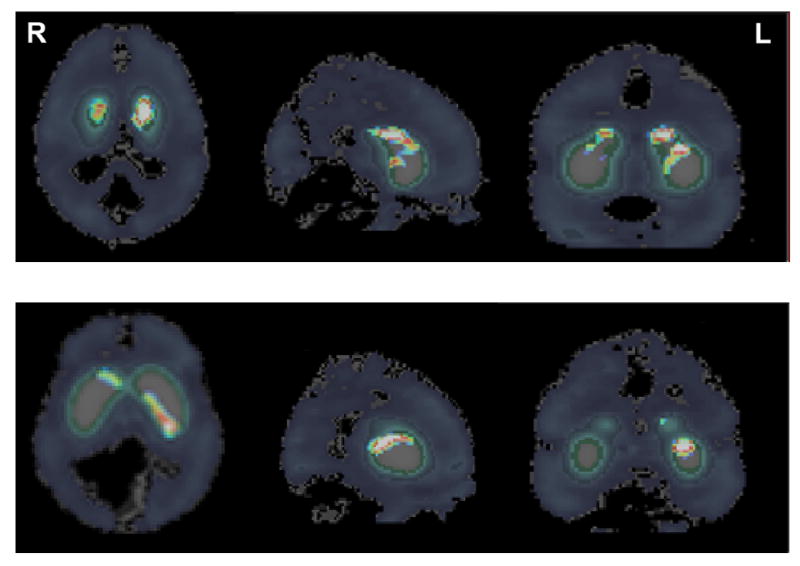
Analyses of the PET data indicated significant decrease in the ligand concentration in several striatal areas after the stimulus presentation was changed from random to sequential (test condition). The decrease in concentration indicated increased rate of ligand displacement from dopamine receptor sites, induced by endogenously released dopamine. To localize the brain areas where the rate of ligand displacement increased significantly, we constructed voxel-wise t-maps using the pooled (Figure 2), and individual (Figure 3) data. The maps show significant task-related displacement in the anterior part of the body of caudate bilaterally (peak t=right 3.25; left 3.42), and in a longitudinal band that spanned posterior two-third of the dorsomedial aspect of left putamen (peak t=3.65).
Figure 2.
SRT Experiment (cohort): The figure shows t-maps indicating group difference in the rate of ligand displacement before and after task initiation. It suggests that the rate increased significantly (t>3.0) after the task initiation in the anterior part of the body of the caudate bilaterally, and in the dorsolateral aspect of posterior two-third of the left putamen.
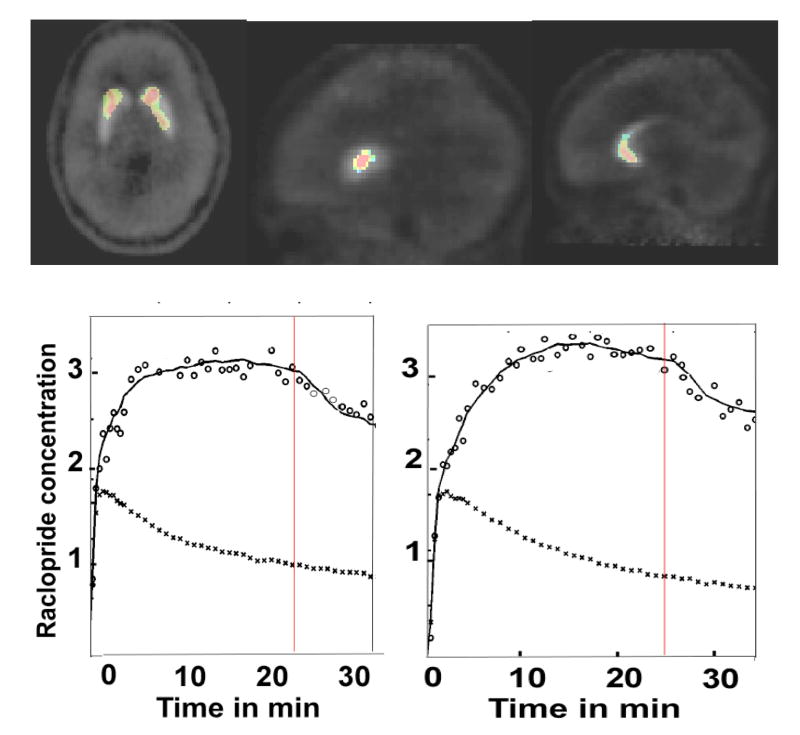
Figure 3.
SRT Experiment (single subject): The pattern of activation observed in the data pooled across subjects (Figure 2), was replicated in individual volunteers. This figure shows t-maps drawn from the data acquired in a volunteer (JW). The time activity curves show the concentration histories (open circles) and least square fits (solid lines) for the ligand (11C-raclopride) in the left anterior caudate (left lower panel) and left posterior putamen (right lower panel). The PET concentration history of the reference region (cerebellum) is also shown (lower curves). The rate of ligand displacement did not change significantly during task performance in this region.
To ensure that the increased rate of ligand displacement was due to receptor ligand interaction and not because of globally increased clearance of the free and nonspecifically bound ligand, we dynamically measured the ligand concentration in a brain region (reference region) that has low density of dopamine receptors (cerebellum). We did not find a significant change in the rate of clearance in this region at any time during the experiment (Figures 2 and 3). This measurement indicated that the changes observed in the striatal areas were due to displacement of the ligand induced by endogenously released dopamine. Further, because the changes were observed in the contrast between the control and test condition, which differed only in the absence (control) or presence (test) of learning related changes, it appears that the changes were induced by the processing associated with sequential learning.
The Control Experiment
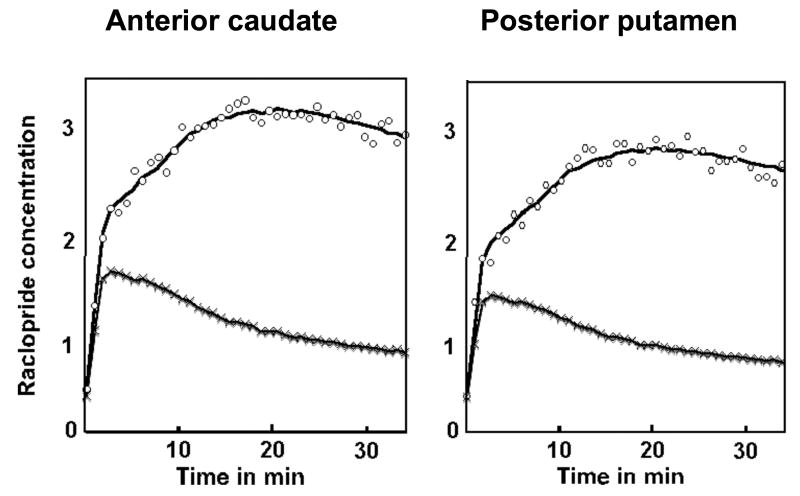
In a separate group of volunteers we conducted a control (no activation) experiment to ensure that the changes observed during sequential learning were not introduced by possible biases in data acquisition and/or analysis. Thus, the acquisition and analysis methods used in this control experiment were exactly same as those used in the SRT experiment. In the control experiment subjects did not perform any task, therefore no additional dopamine release was anticipated. We predicted that there would not be a change in the rate of ligand displacement. As expected, we did not find significant change in any part of the brain (Figure 4). Further, we pooled the PET data of all subjects and constructed a t-map that compared the rate of ligand displacement at different time points. The map did not show significant change in the rate of displacement at any time point in any part of the brain. Absence of a significant change in this experiment confirmed that the changes observed in the SRT experiment were induced by the task, and not by the biases in methods used for data acquisition and analysis.
Figure 4.
Control Experiment: The time activity curves drawn from the left posterior putamen and left anterior caudate (same regions from where curves were drawn in Figure 3) in the control experiment in which subjects did not make any response. There was no significant change in the rate of ligand displacement in this experiment either in the striatal (upper curves) or in the reference region (lower curves).
There is however, a possibility that changes in the rate of ligand displacement observed in the SRT task could be due to nonspecific factors such as, fatigue or general increments in performance. Nonetheless, these factors are not likely to change the rate at a specific time after the task initiation in all volunteers, particularly because the control experiment indicated that the rate remains unchanged for the duration of experiment, if dopamine is not released. Moreover, because the rate of ligand displacement changed almost at the same time when the response time began to change (Figure 1), it appears that the increased rate of ligand displacement was associated with learning related activity.
Further, to ensure that the changes observed in the SRT experiment were not due to changes in the regional cerebral blood flow (rCBF), we conducted a series of simulation studies (Alpert et al., 2003). These studies have indicated that task-induced changes in the rCBF should have no significant effect in this experiment. Based on the previous studies, the SRT task is expected to cause a 2–4% increase of the striatal blood flow (Jueptner et al., 1997). The simulation shows that even a 20% increase will not have a significant effect on the parameters that were used to measure ligand displacement.
Discussion
To our knowledge, this is the first study that has demonstrated endogenous release of striatal dopamine during processing of a learning task. Further, by localizing areas of dopaminergic activity, the experiment provides a novel insight into the striatal and dopaminergic processing of human sequential learning. The finding that dopamine was released in the caudate and left putamen is consistent with the observation of neuroimaging experiments that have reported increased striatal activity during performance of sequential learning tasks (Rauch et al., 1997; Daselaar et al., 2003).
The task-induced release of dopamine in the antero-posterior band that spanned posterior two-third of the dorsomedial aspect of left putamen, suggests that the ‘hand area’ of the striatum was activated during task performance (Gerardin et al., 2004). This band receives motor signals from a number of cortical areas including, the supplementary motor area (SMA), pre-SMA, premotor area (PMA) and the primary motor and sensory cortices (Alexander et al., 1986). In the human striatum, dorsoposterior and posterior putamen (where maximum activation was observed) receive input mostly from the SMA and PMA (Leh et al., 2006). Because of the connectivity with motor areas, it is not surprising that in an earlier experiment we found increased dopamine release along the same band during performance of a motor planning task (Badgaiyan et al., 2003b). In this task, each finger was assigned a number and volunteers were required to tap fingers that corresponded to numerical cues displayed on a computer monitor.
It is interesting that the cortical areas (SMA and PMA) that send signals to the maximally activated part of the putamen (dorsoposterior) are associated with motor learning. Learning related changes in the firing pattern have been recorded from these areas in animals (Brasted and Wise, 2004), and in human volunteers volume of the SMA correlates with performance in the SRT task (Exner et al., 2002). It therefore appears that dopaminergic connectivity between the dorsoposterior putamen, and the SMA and PMA, is associated with the processing of motor sequential learning. This conclusion is in agreement with the findings of an earlier molecular imaging study in which it was observed that the learning related activations of the PMA and VLPFC and the occupancy of striatal dopamine transporter are positively correlated during performance of a sequential motor learning task (Carbon et al., 2004). Further, it appears that the involvement of striatal dopamine extends beyond motor learning because the activated band of putamen also receives input (Lehericy et al., 2004) from the ventrolateral prefrontal cortex (VLPFC), which has previously been implicated in the processing of both, motor (Ungerleider et al., 2002) and nonmotor (Wolf et al., 2006) memory.
In addition to the left putamen, anterior part of the body of the caudate was also activated during sequential learning. Interestingly, this part of the caudate receives input from some of the same cortical areas (e.g., primary motor cortex and VLPFC) from where the activated part of putamen receives inputs (Alexander et al., 1986). These inputs further suggest that dopaminergic control of sequential learning involves the frontostriatal pathways that originate in the PMA and VLPFC. Additional indication of the involvement of these areas comes from neuroimaging studies that have reported increased activity in the VLPFC and PMA during sequential processing (Ungerleider et al., 2002).
A comparison of the present results with those obtained during performance of a motor planning task (Badgaiyan et al., 2003b) reveals an overlap of activated areas in the left putamen and right caudate. Activation in the left caudate however was observed only during sequential learning. This comparison may help us understand how dopaminergic mechanisms process sequential motor learning. It appears that the overlapping activations are associated with the selection and execution of a learned motor response. In both tasks (motor planning and sequential learning) subjects were required to select the finger that was consistent with the instruction (rule), and to make a movement of the selected finger. In this context, the VLPFC appears to play an important role because of its connectivity with both striatal areas (the right caudate and left putamen), where overlapping activities were observed. Further, it has been shown that an important function of the VLPFC is to select a response that is most relevant under a set of rules (Donohue et al., 2005). The activation in these striatal areas therefore, represents the processing associated with the response selection and execution, which was required in both, motor planning and sequential learning task.
In addition to response selection and execution, the sequential learning task required additional processing associated with learning of the sequence and formulation of a new ‘rule’ based on the learned sequence. Since the left caudate was the only additional activation observed in sequential learning (but not in motor planning), it appears that dopamine release in this area was associated with the processing associated with learning and rule formulation.
It has been suggested that the basal ganglia have the capacity of detecting changes in sensory environment or stimulus characteristics (context). These changes are relayed to the cortical structures, which are responsible for making necessary behavioral modifications that are consistent with the altered context (White, 1997). The significance of this communication in learning tasks is suggested by the finding that the learning related changes in neuronal firing occurs in cortical and striatal areas at roughly the same rate, and that these changes match the behavioral rates of sequential learning. In the early phase of sequential learning, changes occur significantly earlier in the striatum (caudate) than in the cortex (Pasupathy and Miller, 2005). Since in the current experiment only the early phase of sequential learning was studied, it appears that the right caudate activity was associated with this learning related activity. Animal experiments indicate that these changes are dopamine dependent. Because of this dependence, the degree of impairment in sequential motor learning tasks correlate with the degree of striatal dopamine depletion (Waelti et al., 2001). Release of striatal dopamine within minutes of the change from random to sequential presentation indicates that dopaminergic mechanisms are involved in the process that allows the striatum to detect a change in the context. This detection occurs before the volunteers are consciously aware of the change and it probably is the first step in the process of learning.
In the present experiment, when stimulus presentation was changed from random to sequential, there was a need to detect the change in ‘context’, and formulate a rule to facilitate response execution. It appears that dopaminergic activation of the left caudate represents the striatal activity associated with this processing, because the activation was observed in the sequential learning task in which the context changed, but not in motor planning task, which did not involve a change in the context. It has been suggested that once the representation of new context is created, the basal ganglia ‘trains’ the frontal cortex to execute new rules that are consistent with the changed context (White, 1997). Since both, the contralateral caudate and VLPFC are associated with the brain mechanisms that are responsible for altering responses following a change in the context (Donohue et al., 2005), it seems plausible that the connectivity between the two structures is associated with the ‘training’ function of the basal ganglia. Further, because sequential processing involves activation of the striatal areas that are associated with motor planning (left putamen and right caudate), it appears that the learning of sequences involves modification of activities of the brain mechanisms involved in the planning of motor movements. This modification may be critical for establishment of sequential learning, and for facilitation of response execution. It therefore appears that the left putamen and right caudate constitute the final common dopaminergic pathway, which is responsible for response execution. It is however not clear from this experiment whether the laterality of activated striatal regions is dependent on the laterality of fingers involved in sequential learning.
Thus, the results demonstrate that the striatal dopamine system is critical for motor sequential learning, and suggest that this form of learning involves two distinct dopaminergic mechanisms. The first mechanism is mediated by the left caudate and is responsible for detection of a change in the ‘context’. The second mechanism involves the left putamen and right caudate and it facilitates response execution by modifying the network that is responsible for planning of motor movements.
Acknowledgments
This work was supported by grants from National Institutes of Health (1R21MH073624), Dana Foundation, and Shriners Hospital for Children (Grant 8580).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alpert NM, Badgaiyan RD, Livini E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. NeuroImage. 2003;19:1049–1060. doi: 10.1016/s1053-8119(03)00186-1. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994a;265:412–45. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 1994b;14:3969–384. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol. 2006;16:213–221. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Detection of task-induced release of extrastriatal dopamine using fallypride. Society of Nuclear Medicine, Journal of Nuclear Medicine. 2007b;48(supp):239–240. [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release during emotional memory processing. Society of Nuclear medicine, Journal of Nuclear Medicine. 2007a;48(supp):240. [Google Scholar]
- Badgaiyan RD, Alpert NM, Fischman AJ. Detection of striatal dopamine released during an explicit motor memory task. Journal of Nuclear Medicine; Annual Meeting of the Society of Nuclear Medicine; 2005. p. 213. [Google Scholar]
- Badgaiyan RD, Alpert NM, Fischman AJ. Neurochemical Mapping of Human Cognition. NeuroImage; Annual Meeting of the Human Brain Mapping Society; 2006a. p. 692. [Google Scholar]
- Badgaiyan RD, Alpert NM, Fischman AJ. Detection of striatal dopamine release during a motor planning task in human volunteers. Brain’03: XXIst International Symposium on Cerebral Blood Flow, Metabolism and Function. J Cerebral Blood Flow and Metabolism. 2003a;23(supp 1):706. [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal dopamine release during unrewarded motor task in human volunteers. Neuroreport. 2003b;14:1421–1424. doi: 10.1097/00001756-200308060-00003. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Detection of striatal dopamine release during an implicit motor memory task. Society of Nuclear Medicine, Journal of Nuclear Medicine. 2004;45(supp 2):9. [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Striatal Dopamine Release During a Cued-Recall Task. Society of Nuclear Medicine, Journal of Nuclear Medicine. 2006b;47(supp 1):138. [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. The role of the basal ganglia in motor control: contributions from PET. J Neurol Sci. 1995;128:1–13. doi: 10.1016/0022-510x(94)00206-4. [DOI] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, Eidelberg D. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004;21:1497–1507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Christian BT, Lehrer DS, Shi B, Narayanan TK, Strohmeyer PS, Buchsbaum MS, Mantil JC. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31:139–152. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–36. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Wendelken C, Crone EA, Bunge SA. Retrieving rules for behavior from long-term memory. Neuroimage. 2005;26:1140–1149. doi: 10.1016/j.neuroimage.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Exner C, Koschack J, Irle E. The differential role of premotor frontal cortex and Basal Ganglia in motor sequence learning: evidence from focal Basal Ganglia lesions. Learn Mem. 2002;9:376–86. doi: 10.1101/lm.48402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Jansson P, Uppfeldt G, Wahlen A, Sedvall G. An open label trial of raclopride in acute schizophrenia. Confirmation of D2-dopamine receptor occupancy by PET. Psychopharmacology. 1988;94:1–7. doi: 10.1007/BF00735871. [DOI] [PubMed] [Google Scholar]
- Fischman AJ, Badgaiyan RD. Neurotrotransmitter Imaging. In: Charron M, editor. Pediatric PET. New York: Springer; 2006. pp. 385–403. [Google Scholar]
- Friston KJ, Malizia AL, Wilson S, Cunningham VJ, Jones T, Nutt DJ. Analysis of dynamic radioligand displacement or "activation" studies. J Cereb Blood Flow Metab. 1997;17:80–93. doi: 10.1097/00004647-199701000-00011. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Pochon JB, Poline JB, Tremblay L, Van de Moortele PF, Levy R, Dubois B, Le Bihan D, Lehericy S. Distinct striatal regions support movement selection, preparation and execution. Neuroreport. 2004;15:2327–2331. doi: 10.1097/00001756-200410250-00005. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor- sequence encoding. A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibanez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121:2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C. Serial reaction time learning and Parkinson’s disease: evidence for a procedural learning deficit. Neuropsychologia. 1995;33:577–593. doi: 10.1016/0028-3932(95)00010-z. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol. 1997;77:1325–137. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36:1182–1190. [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral Mechanisms in Behavior. John Wiley; 1951. [Google Scholar]
- Leh SE, Ptit A, Chakravarty M, Strafella A. Cortical connections of the basal-ganglia: A Probabilistic Diffusion Tractography study. Annual Meeting of the Society of Neuroscience 2006 [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Likert R. A Technique for the Measurement of Attitudes. Archives of Psychology. 1932:140. [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. J Neurophysiol. 1999;82:978–98. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A. In vivo detection of striatal dopamine release during reward: a PET study with [(11)C]raclopride and a single dynamic scan approach. NeuroImage. 2002;16:1015–127. doi: 10.1006/nimg.2002.1121. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Maquet P, Meulemans T, Destrebecqz A, Laureys S, Degueldre C, Delfiore G, Aerts J, Luxen A, Franck G, Van der Linden M, Cleeremans A. Striatum forever, despite sequence learning variability: a random effect analysis of PET data. Hum Brain Mapp. 2000;10:179–194. doi: 10.1002/1097-0193(200008)10:4<179::AID-HBM30>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, Bush G, Breiter HC, Rosen BR. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp. 1997;5:124–132. [PubMed] [Google Scholar]
- Seeman P, Niznik HB, Guan HC, Booth G, Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci U S A. 1989;86:10156–1060. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Schon K, Stern CE. Evidence for the importance of basal ganglia output nuclei in semantic event sequencing: an fMRI study. Brain Res. 2006;1067:239–249. doi: 10.1016/j.brainres.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Van Der Graaf FH, De Jong BM, Maguire RP, Meiners LC, Leenders KL. Cerebral activation related to skills practice in a double serial reaction time task: striatal involvement in random-order sequence learning. Brain Res Cogn Brain Res. 2004;20:120–131. doi: 10.1016/j.cogbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:164–19. doi: 10.1016/s0959-4388(97)80004-9. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Vasic N, Walter H. Differential activation of ventrolateral prefrontal cortex during working memory retrieval. Neuropsychologia. 2006;44:2558–2563. doi: 10.1016/j.neuropsychologia.2006.05.015. [DOI] [PubMed] [Google Scholar]