Abstract
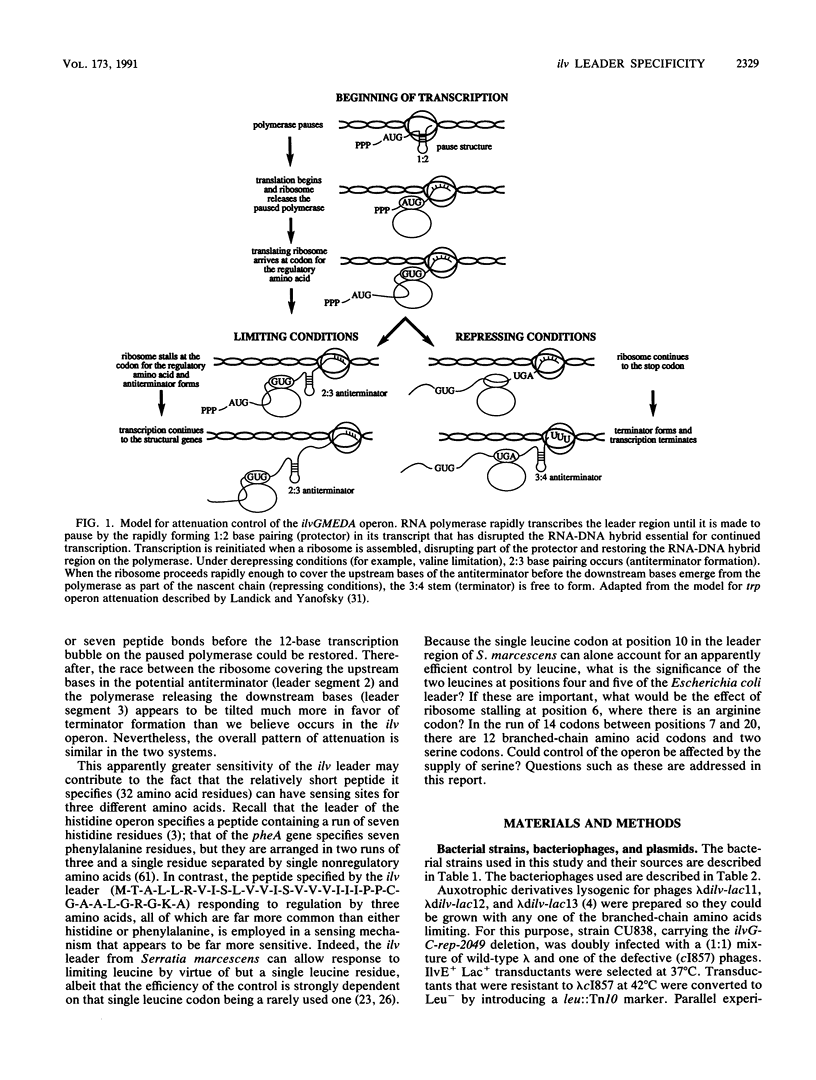
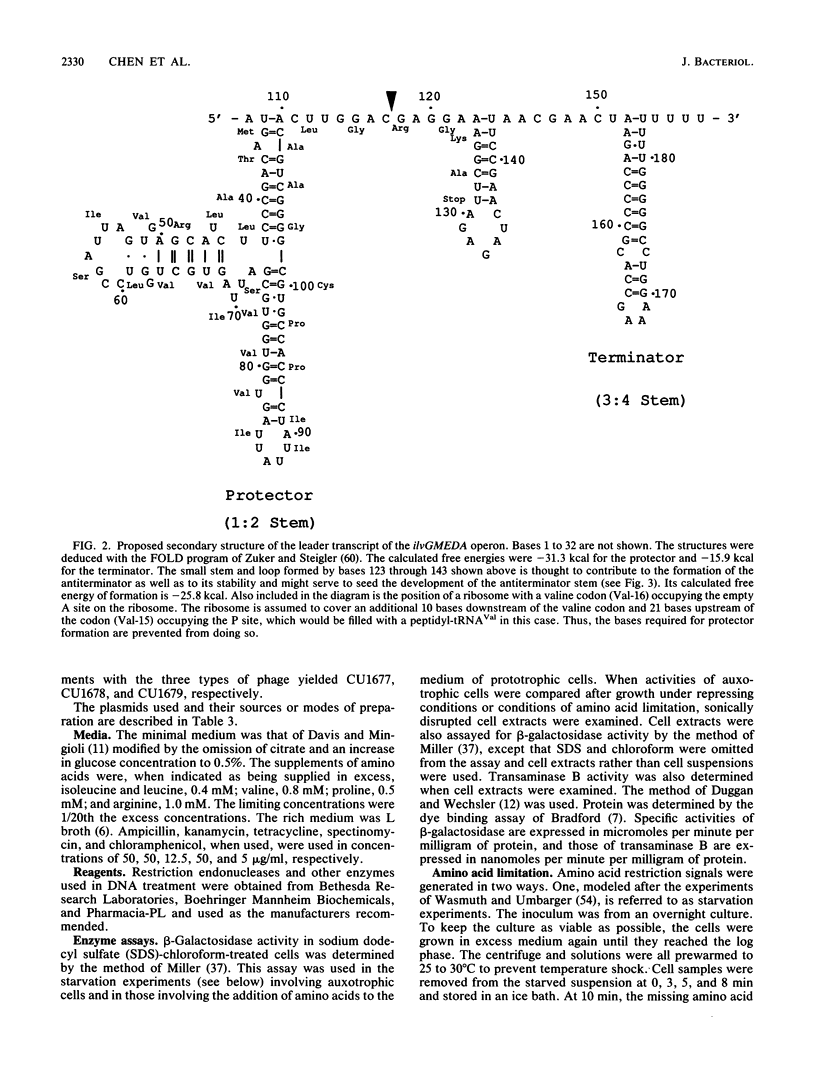
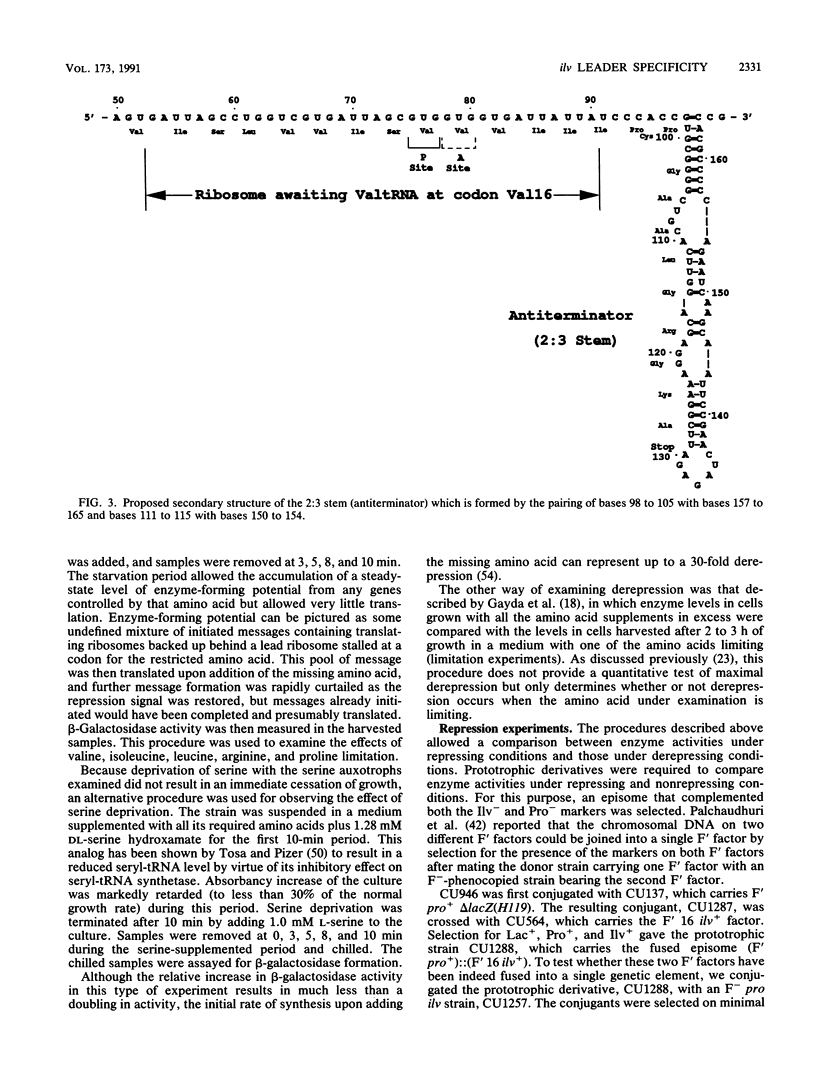
Three different approaches were used to examine the regulatory effects of the amino acids specified by the peptide-coding region of the leader transcript of the ilvGMEDA operon of Escherichia coli K-12. Gene expression was examined in strains carrying an ilvGMED'-lac operon fusion. In one approach, auxotrophic derivatives were starved of single amino acids for brief periods, and the burst of beta-galactosidase synthesis upon adding the missing amino acid was determined. Auxotrophic derivatives were also grown for brief periods with a limited supply of one amino acid (derepression experiments). Finally, prototrophic strains were grown in minimal medium supplemented with single and multiple supplements of the chosen amino acids. Although codons for arginine, serine, and proline are interspersed among the codons for the three branched-chain (regulatory) amino acids, they appeared to have no effect when added in excess to prototrophs or when supplied in restricted amounts to auxotrophs. Deletions removing the terminator stem from the leader removed all ilv-specific control, indicating that the attenuation mechanism is the sole mechanism for ilv-specific control.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Rosenberg M., Hatfield G. W. Analysis of in vivo RNA transcription products of the ilvGEDA attenuator region of Escherichia coli K12. J Biol Chem. 1985 Jul 15;260(14):8538–8544. [PubMed] [Google Scholar]
- Anderson A., Cooper R. A. Biochemical and genetical studies on ribose catabolism in Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):335–339. doi: 10.1099/00221287-62-3-335. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
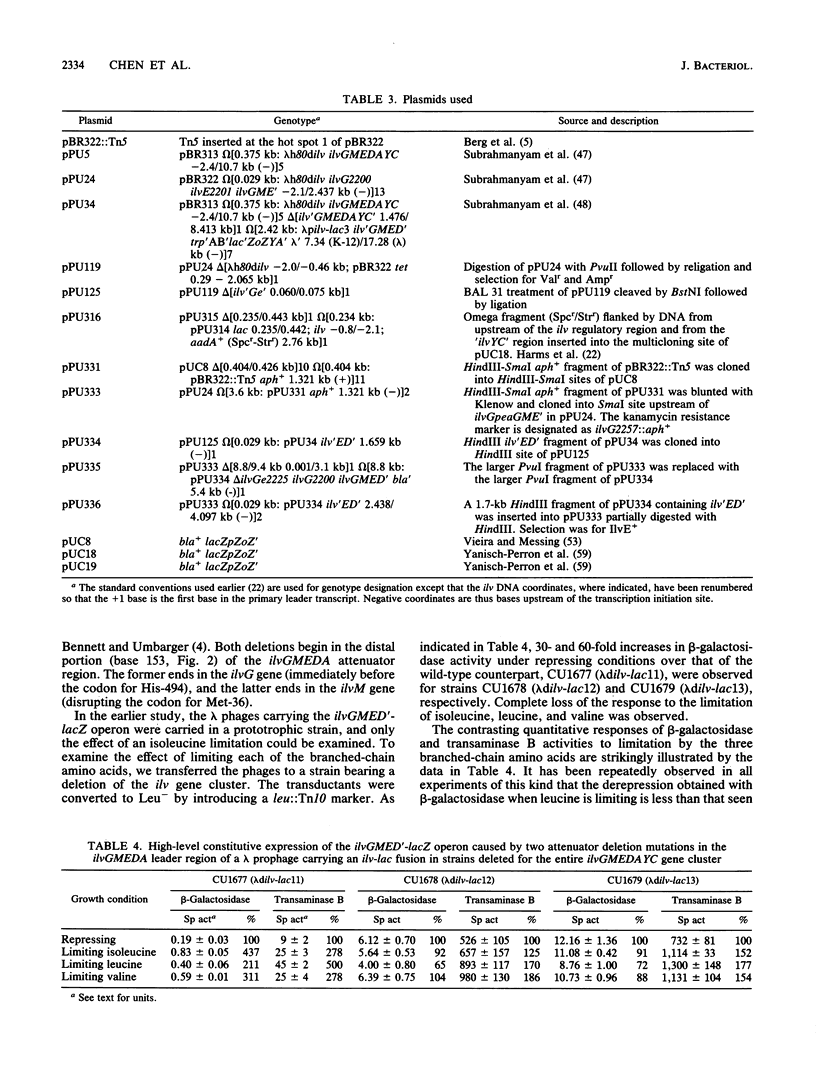
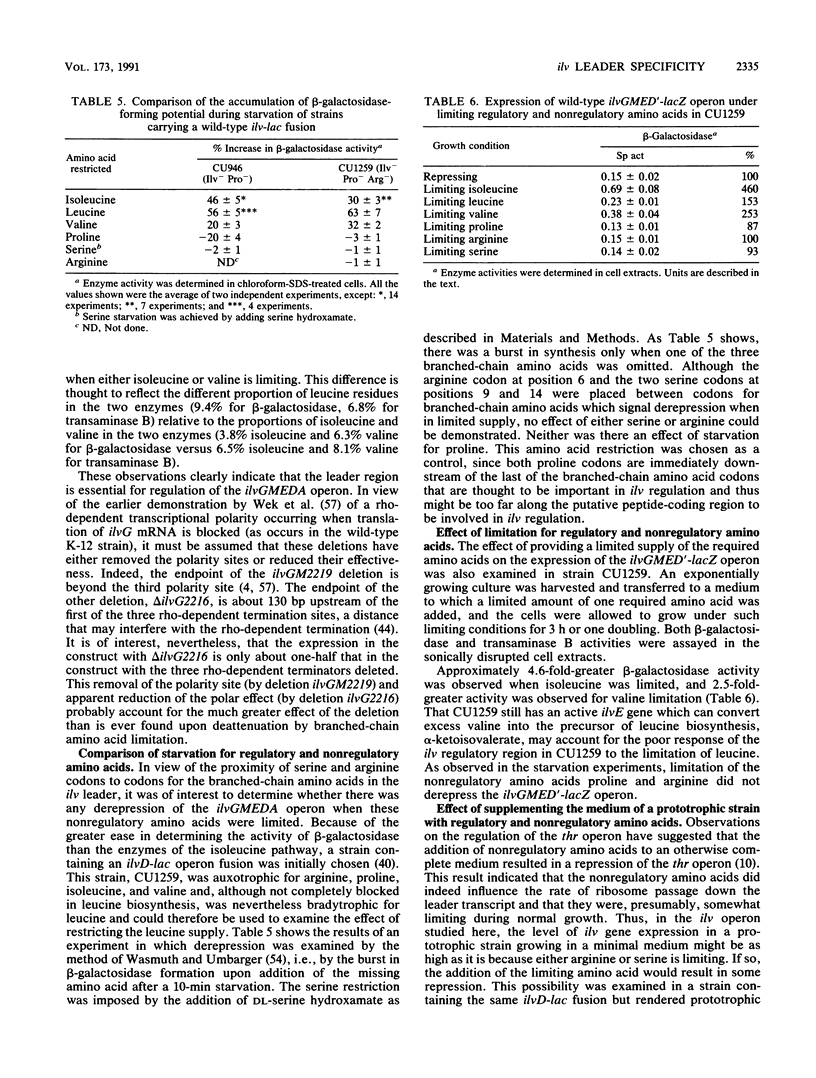
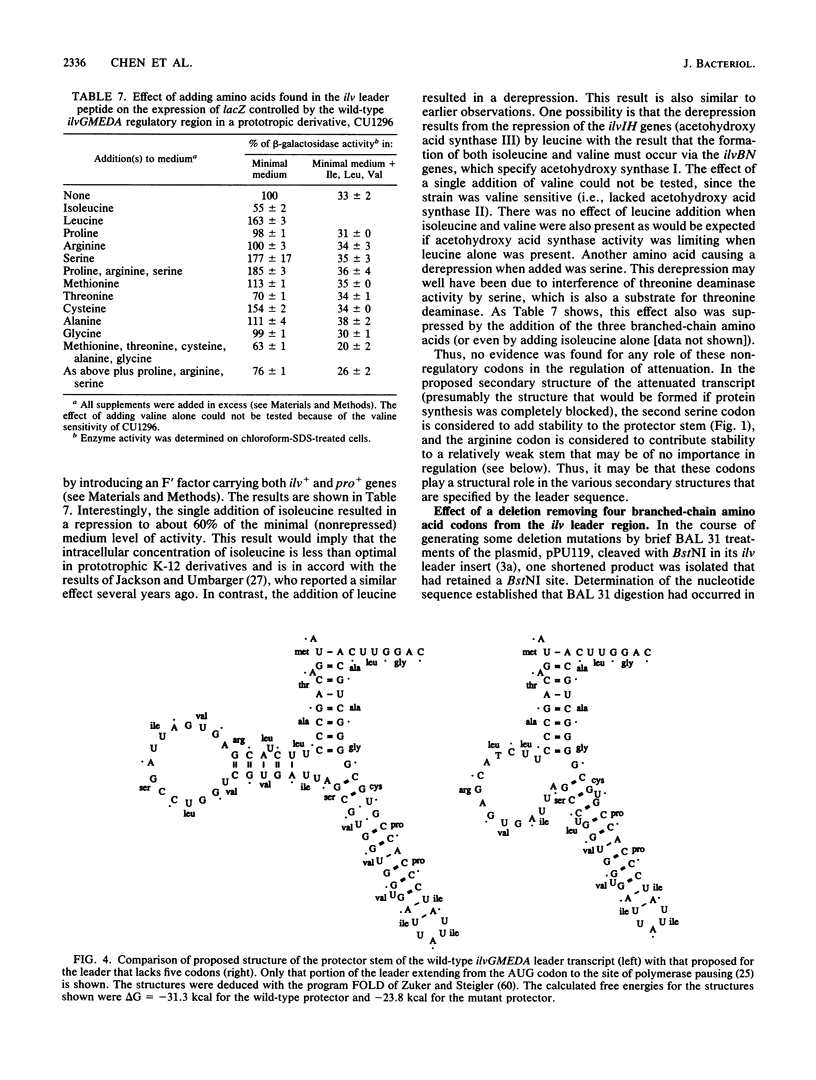
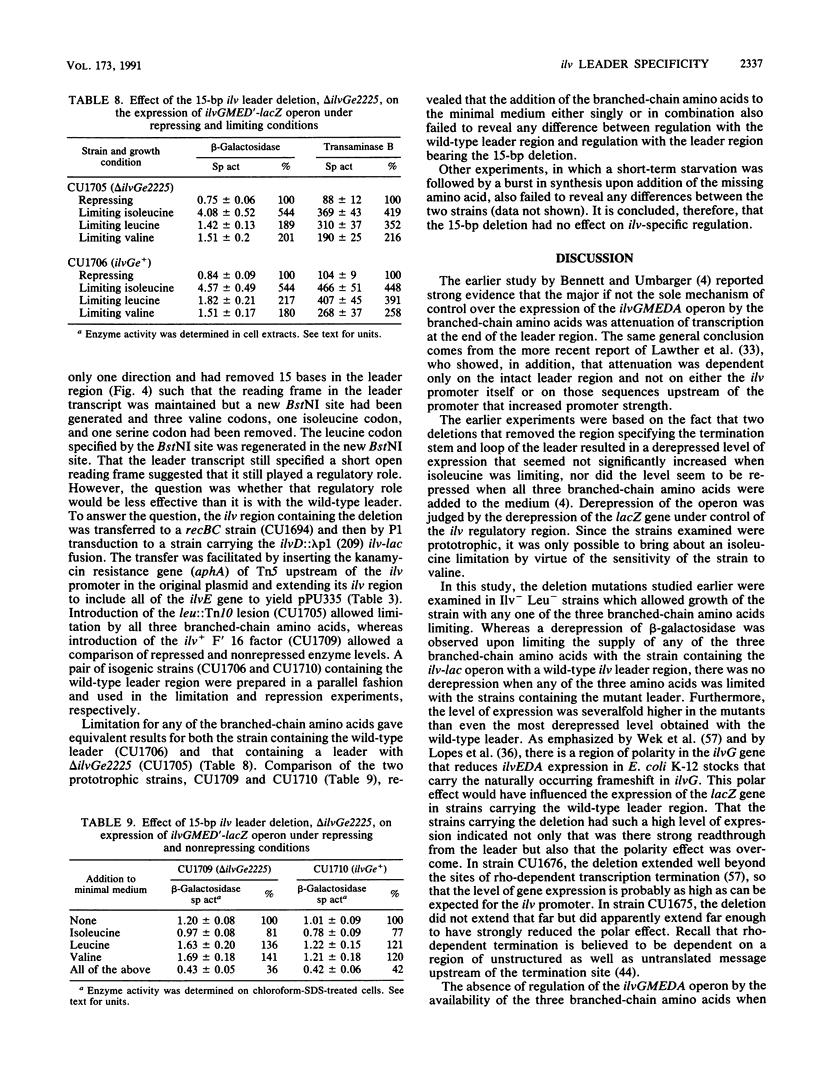
- Bennett D. C., Umbarger H. E. Isolation and analysis of two Escherichia coli K-12 ilv attenuator deletion mutants with high-level constitutive expression of an ilv-lac fusion operon. J Bacteriol. 1984 Mar;157(3):839–845. doi: 10.1128/jb.157.3.839-845.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Schmandt M. A., Lowe J. B. Specificity of transposon Tn5 insertion. Genetics. 1983 Dec;105(4):813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chao L., Vargas C., Spear B. B., Cox E. C. Transposable elements as mutator genes in evolution. Nature. 1983 Jun 16;303(5918):633–635. doi: 10.1038/303633a0. [DOI] [PubMed] [Google Scholar]
- Chen J. W., Harms E., Umbarger H. E. Mutations replacing the leucine codons or altering the length of the amino acid-coding portion of the ilvGMEDA leader region of Escherichia coli. J Bacteriol. 1991 Apr;173(7):2341–2353. doi: 10.1128/jb.173.7.2341-2353.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Saint-Girons I. Attenuation in the threonine operon: effects of amino acids present in the presumed leader peptide in addition to threonine and isoleucine. Mol Gen Genet. 1982;188(2):225–227. doi: 10.1007/BF00332679. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Voelkel K., Sternglanz R., Freundlich M. Reduced expression of the isoleucine and valine enzymes in integration host factor mutants of Escherichia coli. J Mol Biol. 1984 Feb 5;172(4):573–579. doi: 10.1016/s0022-2836(84)80024-8. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. F. Regulation of the threonine operon: tandem threonine and isoleucine codons in the control region and translational control of transcription termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1706–1710. doi: 10.1073/pnas.76.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda D. J., Leathers T. D., Noti J. D., Smith F. J., Smith J. M., Subrahmanyam C. S., Umbarger H. E. Location of the multivalent control site for the ilvEDA operon of Escherichia coli. J Bacteriol. 1980 May;142(2):556–567. doi: 10.1128/jb.142.2.556-567.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollop N., Damri B., Barak Z., Chipman D. M. Kinetics and mechanism of acetohydroxy acid synthase isozyme III from Escherichia coli. Biochemistry. 1989 Jul 25;28(15):6310–6317. doi: 10.1021/bi00441a024. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Harms E., Higgins E., Chen J. W., Umbarger H. E. Translational coupling between the ilvD and ilvA genes of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4798–4807. doi: 10.1128/jb.170.10.4798-4807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms E., Umbarger H. E. Role of codon choice in the leader region of the ilvGMEDA operon of Serratia marcescens. J Bacteriol. 1987 Dec;169(12):5668–5677. doi: 10.1128/jb.169.12.5668-5677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Attenuation of the ilvB operon by amino acids reflecting substrates or products of the ilvB gene product. Proc Natl Acad Sci U S A. 1984 Jan;81(1):76–79. doi: 10.1073/pnas.81.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Sharp J. A., Hatfield L. K., Hatfield G. W. Pausing of RNA polymerase during in vitro transcription through the ilvB and ilvGEDA attenuator regions of Escherichia coli K12. J Biol Chem. 1985 Feb 10;260(3):1765–1770. [PubMed] [Google Scholar]
- Hsu J. H., Harms E., Umbarger H. E. Leucine regulation of the ilvGEDA operon of Serratia marcescens by attenuation is modulated by a single leucine codon. J Bacteriol. 1985 Oct;164(1):217–222. doi: 10.1128/jb.164.1.217-222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. H., Umbarger H. E. Defective transamination, a mechanism for resistance to ketomycin in Escherichia coli. Antimicrob Agents Chemother. 1973 Apr;3(4):510–516. doi: 10.1128/aac.3.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I., Somerville R. L. New regulatory genes involved in the control of transcription initiation at the thr and ilv promoters of Escherichia coli K-12. Mol Gen Genet. 1984;195(1-2):70–76. doi: 10.1007/BF00332726. [DOI] [PubMed] [Google Scholar]
- Kline E. L., Brown C. S., Coleman W. G., Jr, Umbarger H. E. Regulation of isoleucine-valine biosynthesis in an ilvDAC deletion strain of Escherichia coli K-12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1144–1151. doi: 10.1016/0006-291x(74)90816-x. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Lopes J. M., Ortuno M. J., White M. C. Analysis of regulation of the ilvGMEDA operon by using leader-attenuator-galK gene fusions. J Bacteriol. 1990 May;172(5):2320–2327. doi: 10.1128/jb.172.5.2320-2327.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathers T. D., Noti J., Umbarger H. E. Physical characterization of ilv-lac fusions. J Bacteriol. 1979 Oct;140(1):251–260. doi: 10.1128/jb.140.1.251-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. M., Soliman N., Smith P. K., Lawther R. P. Transcriptional polarity enhances the contribution of the internal promoter, ilvEp, in the expression of the ilvGMEDA operon in wild-type Escherichia coli K12. Mol Microbiol. 1989 Aug;3(8):1039–1051. doi: 10.1111/j.1365-2958.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Noti J. D., Umbarger H. E. In vitro formation of beta-galactosidase with a template containing the lac genes fused to gene ilvD. J Bacteriol. 1980 Oct;144(1):291–299. doi: 10.1128/jb.144.1.291-299.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuno M. J., Lawther R. P. Effect of the deletion of upstream DNA sequences on expression from the ilvGp2 promoter of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Feb 25;15(4):1521–1542. doi: 10.1093/nar/15.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchaudhuri S., Maas W. K. Fusion of two F-prime factors in Escherichia coli studied by electron microscope heteroduplex analysis. Mol Gen Genet. 1976 Aug 2;146(3):215–231. doi: 10.1007/BF00701244. [DOI] [PubMed] [Google Scholar]
- Pereira R. F., Ortuno M. J., Lawther R. P. Binding of integration host factor (IHF) to the ilvGp1 promoter of the ilvGMEDA operon of Escherichia coli K12. Nucleic Acids Res. 1988 Jul 11;16(13):5973–5989. doi: 10.1093/nar/16.13.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Rho-dependent transcription termination. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):127–138. doi: 10.1016/0167-4781(90)90048-7. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Ehrlich S. D., Bursztyn H., Lederberg J. Enhancement of transfecting activity of bacteriophage P22 DNA upon exonucleolytic erosion. J Mol Biol. 1976 Aug 25;105(4):587–602. doi: 10.1016/0022-2836(76)90237-0. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam C. S., McCorkle G. M., Umbarger H. E. Physical location of the ilvO determinant in Escherichia coli K-12 deoxyribonucleic acid. J Bacteriol. 1980 May;142(2):547–555. doi: 10.1128/jb.142.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Noti J. D., Umbarger H. E. Regulation of ilvEDA expression occurs upstream of ilvG in Escherichia coli: additional evidence for an ilvGEDA operon. J Bacteriol. 1980 Oct;144(1):279–290. doi: 10.1128/jb.144.1.279-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I., Fassler J. S., Bennett D. C. Relative map location of the rep and rho genes of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1637–1640. doi: 10.1128/jb.151.3.1637-1640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosa T., Pizer L. I. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol. 1971 Jun;106(3):966–971. doi: 10.1128/jb.106.3.966-971.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui P., Freundlich M. Integration host factor binds specifically to sites in the ilvGMEDA operon in Escherichia coli. J Mol Biol. 1988 Oct 5;203(3):817–820. doi: 10.1016/0022-2836(88)90212-4. [DOI] [PubMed] [Google Scholar]
- Tsui P., Freundlich M. Starvation for ilvB operon leader amino acids other than leucine or valine does not increase acetohydroxy acid synthase activity in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1314–1316. doi: 10.1128/jb.162.3.1314-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wasmuth J. J., Umbarger H. E. Effect of isoleucine, valine, or leucine starvation on the potential for formation of the branched-chain amino acid biosynthetic enzymes. J Bacteriol. 1973 Nov;116(2):548–561. doi: 10.1128/jb.116.2.548-561.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. D., Wild J., Umbarger H. E. Positive control of ilvC expression in Escherichia coli K-12; identification and mapping of regulatory gene ilvY. J Bacteriol. 1979 Sep;139(3):1014–1020. doi: 10.1128/jb.139.3.1014-1020.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss D. L., Johnson D. I., Weith H. L., Somerville R. L. Structural analysis of the ileR locus of Escherichia coli K12. J Biol Chem. 1986 Jul 25;261(21):9966–9971. [PubMed] [Google Scholar]
- Wek R. C., Sameshima J. H., Hatfield G. W. Rho-dependent transcriptional polarity in the ilvGMEDA operon of wild-type Escherichia coli K12. J Biol Chem. 1987 Nov 5;262(31):15256–15261. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]


