Abstract
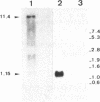
A 24-kb region of Cephalosporium acremonium C10 DNA was cloned by hybridization with the pcbAB and pcbC genes of Penicillium chrysogenum. A 3.2-kb BamHI fragment of this region complemented the mutation in the structural pcbC gene of the C. acremonium N2 mutant, resulting in cephalosporin production. A functional alpha-aminoadipyl-cysteinyl-valine (ACV) synthetase was encoded by a 15.6-kb EcoRI-BamHI DNA fragment, as shown by complementation of an ACV synthetase-deficient mutant of P. chrysogenum. Two transcripts of 1.15 and 11.4 kb were found by Northern (RNA blot) hybridization with probes internal to the pcbC and pcbAB genes, respectively. An open reading frame of 11,136 bp was located upstream of the pcbC gene that matched the 11.4-kb transcript initiation and termination regions. It encoded a protein of 3,712 amino acids with a deduced Mr of 414,791. The nucleotide sequence of the gene showed 62.9% similarity to the pcbAB gene encoding the ACV synthetase of P. chrysogenum; 54.9% of the amino acids were identical in both ACV synthetases. Three highly repetitive regions occur in the deduced amino acid sequence of C. acremonium ACV synthetase. Each is similar to the three repetitive domains in the deduced sequence of P. chrysogenum ACV synthetase and also to the amino acid sequence of gramicidin synthetase I and tyrocidine synthetase I of Bacillus brevis. These regions probably correspond to amino acid activating domains in the ACV synthetase protein. In addition, a thioesterase domain was present in the ACV synthetases of both fungi. A similarity has been found between the domains existing in multienzyme nonribosomal peptide synthetases and polyketide and fatty acid synthetases. The pcbAB gene is linked to the pcbC gene, forming a cluster of early cephalosporin-biosynthetic genes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. E., Bird J. W., Field R. A., O'Callaghan N. M., Schofield C. J. Isolation and partial characterisation of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus. J Antibiot (Tokyo) 1990 Aug;43(8):1055–1057. doi: 10.7164/antibiotics.43.1055. [DOI] [PubMed] [Google Scholar]
- Ballance D. J. Sequences important for gene expression in filamentous fungi. Yeast. 1986 Dec;2(4):229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- Banko G., Wolfe S., Demain A. L. Cell-free synthesis of delta-(L-alpha-aminoadipyl)-L-cysteine, the first intermediate of penicillin and cephalosporin biosynthesis. Biochem Biophys Res Commun. 1986 May 29;137(1):528–535. doi: 10.1016/0006-291x(86)91242-8. [DOI] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Barredo J. L., Cantoral J. M., Alvarez E., Díez B., Martín J. F. Cloning, sequence analysis and transcriptional study of the isopenicillin N synthase of Penicillium chrysogenum AS-P-78. Mol Gen Genet. 1989 Mar;216(1):91–98. doi: 10.1007/BF00332235. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Díez B., Barredo J. L., Alvarez E., Cantoral J. M., van Solingen P., Groenen M. A., Veenstra A. E., Martín J. F. Two genes involved in penicillin biosynthesis are linked in a 5.1 kb SalI fragment in the genome of Penicillium chrysogenum. Mol Gen Genet. 1989 Sep;218(3):572–576. doi: 10.1007/BF00332426. [DOI] [PubMed] [Google Scholar]
- Díez B., Gutiérrez S., Barredo J. L., van Solingen P., van der Voort L. H., Martín J. F. The cluster of penicillin biosynthetic genes. Identification and characterization of the pcbAB gene encoding the alpha-aminoadipyl-cysteinyl-valine synthetase and linkage to the pcbC and penDE genes. J Biol Chem. 1990 Sep 25;265(27):16358–16365. [PubMed] [Google Scholar]
- Eriquez L. A., Pisano M. A. Isolation and nature of intracellular alpha-aminoadipic acid-containing peptides from Paecilomyces persicinus P-10. Antimicrob Agents Chemother. 1979 Sep;16(3):392–397. doi: 10.1128/aac.16.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett P. A., Usher J. J., Huddleston J. A., Bleaney R. C., Nisbet J. J., Abraham E. P. Synthesis of delta-(alpha-aminoadipyl)cysteinylvaline and its role in penicillin biosynthesis. Biochem J. 1976 Sep 1;157(3):651–660. doi: 10.1042/bj1570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushima H., Yasuda S., Soeda E., Yokota M., Kondo M., Kimura A. Complete nucleotide sequence of the E. coli glutathione synthetase gsh-II. Nucleic Acids Res. 1984 Dec 21;12(24):9299–9307. doi: 10.1093/nar/12.24.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D. G., Dewart K. B., Aitken A., McCarthy A. D. Amino acid sequence around the reactive serine residue of the thioesterase domain of rabbit fatty acid synthase. Biochim Biophys Acta. 1985 Apr 29;828(3):380–382. doi: 10.1016/0167-4838(85)90320-6. [DOI] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Biosynthesis of peptide antibiotics. Annu Rev Microbiol. 1987;41:259–289. doi: 10.1146/annurev.mi.41.100187.001355. [DOI] [PubMed] [Google Scholar]
- Krause M., Marahiel M. A. Organization of the biosynthesis genes for the peptide antibiotic gramicidin S. J Bacteriol. 1988 Oct;170(10):4669–4674. doi: 10.1128/jb.170.10.4669-4674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipmann F. Attempts to map a process evolution of peptide biosynthesis. Science. 1971 Sep 3;173(4000):875–884. doi: 10.1126/science.173.4000.875. [DOI] [PubMed] [Google Scholar]
- MacCabe A. P., Riach M. B., Unkles S. E., Kinghorn J. R. The Aspergillus nidulans npeA locus consists of three contiguous genes required for penicillin biosynthesis. EMBO J. 1990 Jan;9(1):279–287. doi: 10.1002/j.1460-2075.1990.tb08106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín J. F. Biosynthesis of polyene macrolide antibiotics. Annu Rev Microbiol. 1977;31:13–38. doi: 10.1146/annurev.mi.31.100177.000305. [DOI] [PubMed] [Google Scholar]
- Martín J. F., Liras P. Enzymes involved in penicillin, cephalosporin and cephamycin biosynthesis. Adv Biochem Eng Biotechnol. 1989;39:153–187. doi: 10.1007/BFb0051954. [DOI] [PubMed] [Google Scholar]
- Martín M. F., Liras P. Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol. 1989;43:173–206. doi: 10.1146/annurev.mi.43.100189.001133. [DOI] [PubMed] [Google Scholar]
- Mittenhuber G., Weckermann R., Marahiel M. A. Gene cluster containing the genes for tyrocidine synthetases 1 and 2 from Bacillus brevis: evidence for an operon. J Bacteriol. 1989 Sep;171(9):4881–4887. doi: 10.1128/jb.171.9.4881-4887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro E., Barredo J. L., Gutiérrez S., Díez B., Alvarez E., Martín J. F. Cloning, characterization of the acyl-CoA:6-amino penicillanic acid acyltransferase gene of Aspergillus nidulans and linkage to the isopenicillin N synthase gene. Mol Gen Genet. 1990 May;221(3):322–330. doi: 10.1007/BF00259395. [DOI] [PubMed] [Google Scholar]
- Naggert J., Witkowski A., Mikkelsen J., Smith S. Molecular cloning and sequencing of a cDNA encoding the thioesterase domain of the rat fatty acid synthetase. J Biol Chem. 1988 Jan 25;263(3):1146–1150. [PubMed] [Google Scholar]
- Poulose A. J., Rogers L., Kolattukudy P. E. Primary structure of a chymotryptic peptide containing the "active serine" of the thioesterase domain of fatty acid synthase. Biochem Biophys Res Commun. 1981 Nov 30;103(2):377–382. doi: 10.1016/0006-291x(81)90463-0. [DOI] [PubMed] [Google Scholar]
- Ramsden M., McQuade B. A., Saunders K., Turner M. K., Harford S. Characterization of a loss-of-function mutation in the isopenicillin N synthetase gene of Acremonium chrysogenum. Gene. 1989 Dec 21;85(1):267–273. doi: 10.1016/0378-1119(89)90493-9. [DOI] [PubMed] [Google Scholar]
- Randhawa Z. I., Naggert J., Blacher R. W., Smith S. Amino acid sequence of the serine active-site region of the medium-chain S-acyl fatty acid synthetase thioester hydrolase from rat mammary gland. Eur J Biochem. 1987 Feb 2;162(3):577–581. doi: 10.1111/j.1432-1033.1987.tb10678.x. [DOI] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer E., Werkmeister K., Jain M. K. Fatty acid biosynthesis in yeast. Mol Cell Biochem. 1978 Nov 1;21(2):95–107. doi: 10.1007/BF00240280. [DOI] [PubMed] [Google Scholar]
- Skatrud P. L., Queener S. W. An electrophoretic molecular karyotype for an industrial strain of Cephalosporium acremonium. Gene. 1989 May 30;78(2):331–338. doi: 10.1016/0378-1119(89)90235-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yamano Y., Murata K., Kimura A. The nucleotide sequence of the gene for gamma-glutamylcysteine synthetase of Escherichia coli. Nucleic Acids Res. 1986 Jun 11;14(11):4393–4400. doi: 10.1093/nar/14.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z. Y., Liu W., Hammes G. G. Molecular cloning and sequencing of DNA complementary to chicken liver fatty acid synthase mRNA. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6328–6331. doi: 10.1073/pnas.85.17.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt H., von Döhren H., Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989 Mar 5;264(7):3680–3684. [PubMed] [Google Scholar]